[English] 日本語
 Yorodumi
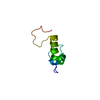
Yorodumi- PDB-1i8g: SOLUTION STRUCTURE OF PIN1 WW DOMAIN COMPLEXED WITH CDC25 PHOSPHO... -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 1i8g | ||||||
|---|---|---|---|---|---|---|---|
| Title | SOLUTION STRUCTURE OF PIN1 WW DOMAIN COMPLEXED WITH CDC25 PHOSPHOTHREONINE PEPTIDE | ||||||
 Components Components |
| ||||||
 Keywords Keywords | HYDROLASE/ISOMERASE / CELL DIVISION / NUCLEAR PROTEIN / HYDROLASE-ISOMERASE COMPLEX | ||||||
| Function / homology |  Function and homology information Function and homology informationpositive regulation of G2/MI transition of meiotic cell cycle / cis-trans isomerase activity / phosphothreonine residue binding / negative regulation of cell motility / ubiquitin ligase activator activity / regulation of protein localization to nucleus / GTPase activating protein binding / mitogen-activated protein kinase kinase binding / protein targeting to mitochondrion / protein peptidyl-prolyl isomerization ...positive regulation of G2/MI transition of meiotic cell cycle / cis-trans isomerase activity / phosphothreonine residue binding / negative regulation of cell motility / ubiquitin ligase activator activity / regulation of protein localization to nucleus / GTPase activating protein binding / mitogen-activated protein kinase kinase binding / protein targeting to mitochondrion / protein peptidyl-prolyl isomerization / regulation of mitotic nuclear division / negative regulation of SMAD protein signal transduction / PI5P Regulates TP53 Acetylation / negative regulation of amyloid-beta formation / cytoskeletal motor activity / RHO GTPases Activate NADPH Oxidases / phosphoserine residue binding / positive regulation of G2/M transition of mitotic cell cycle / postsynaptic cytosol / negative regulation of protein binding / Rho protein signal transduction / protein-tyrosine-phosphatase / protein tyrosine phosphatase activity / regulation of cytokinesis / peptidylprolyl isomerase / Negative regulators of DDX58/IFIH1 signaling / peptidyl-prolyl cis-trans isomerase activity / phosphoprotein binding / negative regulation of transforming growth factor beta receptor signaling pathway / beta-catenin binding / synapse organization / negative regulation of protein catabolic process / regulation of protein stability / negative regulation of ERK1 and ERK2 cascade / ISG15 antiviral mechanism / G2/M transition of mitotic cell cycle / tau protein binding / positive regulation of protein phosphorylation / neuron differentiation / positive regulation of canonical Wnt signaling pathway / regulation of gene expression / midbody / cellular response to hypoxia / Regulation of TP53 Activity through Phosphorylation / response to hypoxia / protein stabilization / nuclear speck / ciliary basal body / cell division / glutamatergic synapse / positive regulation of transcription by RNA polymerase II / nucleoplasm / nucleus / cytosol / cytoplasm Similarity search - Function | ||||||
| Method | SOLUTION NMR / distance geometry simulated annealing | ||||||
 Authors Authors | Wintjens, R. / Wieruszeski, J.-M. / Drobecq, H. / Lippens, G. / Landrieu, I. | ||||||
 Citation Citation |  Journal: J.Biol.Chem. / Year: 2001 Journal: J.Biol.Chem. / Year: 2001Title: 1H NMR study on the binding of Pin1 Trp-Trp domain with phosphothreonine peptides. Authors: Wintjens, R. / Wieruszeski, J.M. / Drobecq, H. / Rousselot-Pailley, P. / Buee, L. / Lippens, G. / Landrieu, I. | ||||||
| History |
|
- Structure visualization
Structure visualization
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
|---|
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  1i8g.cif.gz 1i8g.cif.gz | 162.6 KB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb1i8g.ent.gz pdb1i8g.ent.gz | 133.9 KB | Display |  PDB format PDB format |
| PDBx/mmJSON format |  1i8g.json.gz 1i8g.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Summary document |  1i8g_validation.pdf.gz 1i8g_validation.pdf.gz | 366.8 KB | Display |  wwPDB validaton report wwPDB validaton report |
|---|---|---|---|---|
| Full document |  1i8g_full_validation.pdf.gz 1i8g_full_validation.pdf.gz | 460.2 KB | Display | |
| Data in XML |  1i8g_validation.xml.gz 1i8g_validation.xml.gz | 11.8 KB | Display | |
| Data in CIF |  1i8g_validation.cif.gz 1i8g_validation.cif.gz | 18.1 KB | Display | |
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/i8/1i8g https://data.pdbj.org/pub/pdb/validation_reports/i8/1i8g ftp://data.pdbj.org/pub/pdb/validation_reports/i8/1i8g ftp://data.pdbj.org/pub/pdb/validation_reports/i8/1i8g | HTTPS FTP |
-Related structure data
- Links
Links
- Assembly
Assembly
| Deposited unit | 
| |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 1 |
| |||||||||
| NMR ensembles |
|
- Components
Components
| #1: Protein/peptide | Mass: 1192.209 Da / Num. of mol.: 1 / Fragment: RESIDUES 63-72 / Source method: obtained synthetically Details: The ligand phosphopeptide was synthesized from Rink amide resin using the Fmoc strategy and activation by HBTU and HOBT in a 431A peptide synthesizer. The sequence of the peptide is ...Details: The ligand phosphopeptide was synthesized from Rink amide resin using the Fmoc strategy and activation by HBTU and HOBT in a 431A peptide synthesizer. The sequence of the peptide is naturally found in Xenopus laevis (African clawed frog). References: UniProt: P30311, protein-tyrosine-phosphatase |
|---|---|
| #2: Protein/peptide | Mass: 4462.899 Da / Num. of mol.: 1 / Fragment: WW DOMAIN (RESIDUES 6-44) / Source method: obtained synthetically Details: The Pin1 WW domain was obtained by peptide synthesis using the BOC-benzyl strategy and the HBTU in situ activation protocol on an Applied 430A peptide synthesizer. The sequence of the ...Details: The Pin1 WW domain was obtained by peptide synthesis using the BOC-benzyl strategy and the HBTU in situ activation protocol on an Applied 430A peptide synthesizer. The sequence of the peptide is naturally found in Homo sapiens (Human). References: UniProt: Q13526, peptidylprolyl isomerase |
| Has protein modification | Y |
-Experimental details
-Experiment
| Experiment | Method: SOLUTION NMR |
|---|---|
| NMR experiment | Type: 2D NOESY |
- Sample preparation
Sample preparation
| Details | Contents: sample of 1mM WW domain / 4.5 mM Cdc25 ligand buffer of 50 mM deutered Tris-D2O, pH 6.4, 100 mM NaCl Solvent system: 90% H2O/10% D2O |
|---|---|
| Sample conditions | Ionic strength: 100 mM NaCl / pH: 6.4 / Pressure: ambient / Temperature: 285 K |
| Crystal grow | *PLUS Method: other / Details: NMR |
-NMR measurement
| NMR spectrometer | Type: Bruker DMX / Manufacturer: Bruker / Model: DMX / Field strength: 600 MHz |
|---|
- Processing
Processing
| NMR software |
| ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Refinement | Method: distance geometry simulated annealing / Software ordinal: 1 Details: hybrid of distance geometry / simulated annealing protocol Minimization procedure using CVFF as force field | ||||||||||||
| NMR representative | Selection criteria: closest to the average | ||||||||||||
| NMR ensemble | Conformer selection criteria: structures with the lowest energy Conformers calculated total number: 50 / Conformers submitted total number: 10 |
 Movie
Movie Controller
Controller









 PDBj
PDBj







 X-PLOR
X-PLOR