[English] 日本語
 Yorodumi
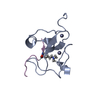
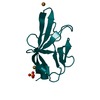
Yorodumi- PDB-1i1j: STRUCTURE OF MELANOMA INHIBITORY ACTIVITY PROTEIN: A MEMBER OF A ... -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 1i1j | ||||||
|---|---|---|---|---|---|---|---|
| Title | STRUCTURE OF MELANOMA INHIBITORY ACTIVITY PROTEIN: A MEMBER OF A NEW FAMILY OF SECRETED PROTEINS | ||||||
 Components Components | MELANOMA DERIVED GROWTH REGULATORY PROTEIN | ||||||
 Keywords Keywords | HORMONE/GROWTH FACTOR / SH3 subdomain / HORMONE-GROWTH FACTOR COMPLEX | ||||||
| Function / homology |  Function and homology information Function and homology informationextracellular matrix organization / growth factor activity / extracellular space Similarity search - Function | ||||||
| Biological species |  Homo sapiens (human) Homo sapiens (human) | ||||||
| Method |  X-RAY DIFFRACTION / X-RAY DIFFRACTION /  SYNCHROTRON / SYNCHROTRON /  MAD / Resolution: 1.39 Å MAD / Resolution: 1.39 Å | ||||||
 Authors Authors | Lougheed, J.C. / Holton, J.M. / Alber, T. / Bazan, J.F. / Handel, T.M. | ||||||
 Citation Citation |  Journal: Proc.Natl.Acad.Sci.USA / Year: 2001 Journal: Proc.Natl.Acad.Sci.USA / Year: 2001Title: Structure of melanoma inhibitory activity protein, a member of a recently identified family of secreted proteins. Authors: Lougheed, J.C. / Holton, J.M. / Alber, T. / Bazan, J.F. / Handel, T.M. | ||||||
| History |
|
- Structure visualization
Structure visualization
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
|---|
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  1i1j.cif.gz 1i1j.cif.gz | 57.9 KB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb1i1j.ent.gz pdb1i1j.ent.gz | 42.1 KB | Display |  PDB format PDB format |
| PDBx/mmJSON format |  1i1j.json.gz 1i1j.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/i1/1i1j https://data.pdbj.org/pub/pdb/validation_reports/i1/1i1j ftp://data.pdbj.org/pub/pdb/validation_reports/i1/1i1j ftp://data.pdbj.org/pub/pdb/validation_reports/i1/1i1j | HTTPS FTP |
|---|
-Related structure data
| Similar structure data |
|---|
- Links
Links
- Assembly
Assembly
| Deposited unit | 
| ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 1 | 
| ||||||||
| 2 | 
| ||||||||
| Unit cell |
|
- Components
Components
| #1: Protein | Mass: 12258.138 Da / Num. of mol.: 2 Source method: isolated from a genetically manipulated source Source: (gene. exp.)  Homo sapiens (human) / Production host: Homo sapiens (human) / Production host:  #2: Water | ChemComp-HOH / | Has protein modification | Y | |
|---|
-Experimental details
-Experiment
| Experiment | Method:  X-RAY DIFFRACTION / Number of used crystals: 2 X-RAY DIFFRACTION / Number of used crystals: 2 |
|---|
- Sample preparation
Sample preparation
| Crystal | Density Matthews: 2.06 Å3/Da / Density % sol: 40.23 % | ||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Crystal grow | Temperature: 295 K / Method: vapor diffusion, hanging drop / pH: 8.2 Details: PEG 8000, Tris, sodium chloride, pH 8.2, VAPOR DIFFUSION, HANGING DROP, temperature 295K | ||||||||||||||||||||||||||||||
| Crystal grow | *PLUS pH: 4.55 | ||||||||||||||||||||||||||||||
| Components of the solutions | *PLUS
|
-Data collection
| Diffraction |
| ||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Diffraction source |
| ||||||||||||||||||||||||
| Detector |
| ||||||||||||||||||||||||
| Radiation |
| ||||||||||||||||||||||||
| Radiation wavelength |
| ||||||||||||||||||||||||
| Reflection | Resolution: 1.39→43.85 Å / Num. all: 47146 / Num. obs: 47146 / % possible obs: 97.76 % / Observed criterion σ(F): -3 / Observed criterion σ(I): -3 / Redundancy: 6.6 % / Biso Wilson estimate: 18.141 Å2 / Rmerge(I) obs: 0.062 / Rsym value: 0.062 / Net I/σ(I): 16.8 | ||||||||||||||||||||||||
| Reflection shell | Resolution: 1.39→1.47 Å / Redundancy: 2.9 % / Rmerge(I) obs: 0.24 / Mean I/σ(I) obs: 3 / Num. unique all: 15627 / Rsym value: 0.24 / % possible all: 91.5 | ||||||||||||||||||||||||
| Reflection | *PLUS % possible obs: 99.44 % |
- Processing
Processing
| Software |
| |||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Refinement | Method to determine structure:  MAD / Resolution: 1.39→43.85 Å / Isotropic thermal model: Isotropic / Cross valid method: THROUGHOUT / σ(F): -3 / σ(I): -3 / Stereochemistry target values: Engh & Huber 1991 MAD / Resolution: 1.39→43.85 Å / Isotropic thermal model: Isotropic / Cross valid method: THROUGHOUT / σ(F): -3 / σ(I): -3 / Stereochemistry target values: Engh & Huber 1991
| |||||||||||||||||||||||||
| Refinement step | Cycle: LAST / Resolution: 1.39→43.85 Å
| |||||||||||||||||||||||||
| Refine LS restraints |
| |||||||||||||||||||||||||
| Software | *PLUS Name: REFMAC / Classification: refinement | |||||||||||||||||||||||||
| Refinement | *PLUS σ(F): -3 | |||||||||||||||||||||||||
| Solvent computation | *PLUS | |||||||||||||||||||||||||
| Displacement parameters | *PLUS Biso mean: 23.35 Å2 |
 Movie
Movie Controller
Controller










 PDBj
PDBj

