[English] 日本語
 Yorodumi
Yorodumi- PDB-1haj: A beta-Hairpin Structure in a 13-mer Peptide that Binds a-Bungaro... -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 1haj | ||||||
|---|---|---|---|---|---|---|---|
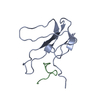
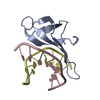
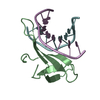
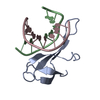
| Title | A beta-Hairpin Structure in a 13-mer Peptide that Binds a-Bungarotoxin with High Affinity and Neutralizes its Toxicity | ||||||
 Components Components |
| ||||||
 Keywords Keywords | TOXIN/PEPTIDE / COMPLEX (TOXIN-PEPTIDE) / ACETYLCHOLINE RECEPTOR MIMITOPE / ALPHA-BUNGAROTOXIN / PROTEIN-PEPTIDE COMPLEX / TOXIN / BETA-HAIRPIN / TOXIN-PEPTIDE complex | ||||||
| Function / homology |  Function and homology information Function and homology informationacetylcholine receptor inhibitor activity / ion channel regulator activity / toxin activity / extracellular region Similarity search - Function | ||||||
| Biological species |  BUNGARUS MULTICINCTUS (many-banded krait) BUNGARUS MULTICINCTUS (many-banded krait)SYNTHETIC CONSTRUCT (others) | ||||||
| Method | SOLUTION NMR / DISTANCE GEOMETRY, DYNAMICAL SIMULATED ANNEALING | ||||||
 Authors Authors | Scherf, T. / Kasher, R. / Balass, M. / Fridkin, M. / Fuchs, S. / Katchalski-Katzir, E. | ||||||
 Citation Citation |  Journal: Proc.Natl.Acad.Sci.USA / Year: 2001 Journal: Proc.Natl.Acad.Sci.USA / Year: 2001Title: A Beta-Hairpin Structure in a 13-mer Peptide that Binds Alpha-Bungarotoxin with High Affinity and Neutralizes its Toxicity Authors: Scherf, T. / Kasher, R. / Balass, M. / Fridkin, M. / Fuchs, S. / Katchalski-Katzir, E. #1: Journal: Chem.Biol. / Year: 2001 Title: Design and Synthesis of Peptides that Bind A-Bungarotoxin with High Affinity Authors: Kasher, R. / Balass, M. / Scherf, T. / Fridkin, M. / Fuchs, S. / Katchalski-Katzir, E. | ||||||
| History |
| ||||||
| Remark 700 | SHEET DETERMINATION METHOD: KABSCH AND SANDER ALGORITHM STRAND: S1A 1; STRANDS S2D & S2E FORM B- ... SHEET DETERMINATION METHOD: KABSCH AND SANDER ALGORITHM STRAND: S1A 1; STRANDS S2D & S2E FORM B-HAIRPIN WITHIN THE BOUND PEPTIDE, THAT COMBINES TO S2A-S2C TO FORM 5-STRANDED INTERMOLECULAR BETA-SHEET. |
- Structure visualization
Structure visualization
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
|---|
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  1haj.cif.gz 1haj.cif.gz | 262.4 KB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb1haj.ent.gz pdb1haj.ent.gz | 215.6 KB | Display |  PDB format PDB format |
| PDBx/mmJSON format |  1haj.json.gz 1haj.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/ha/1haj https://data.pdbj.org/pub/pdb/validation_reports/ha/1haj ftp://data.pdbj.org/pub/pdb/validation_reports/ha/1haj ftp://data.pdbj.org/pub/pdb/validation_reports/ha/1haj | HTTPS FTP |
|---|
-Related structure data
- Links
Links
- Assembly
Assembly
| Deposited unit | 
| |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 1 |
| |||||||||
| NMR ensembles |
|
- Components
Components
| #1: Protein | Mass: 8005.281 Da / Num. of mol.: 1 / Source method: isolated from a natural source / Details: ALPHA-NEUROTOXIN / Source: (natural)  BUNGARUS MULTICINCTUS (many-banded krait) / Secretion: VENOM / References: UniProt: P01378, UniProt: P60615*PLUS BUNGARUS MULTICINCTUS (many-banded krait) / Secretion: VENOM / References: UniProt: P01378, UniProt: P60615*PLUS |
|---|---|
| #2: Protein/peptide | Mass: 1705.799 Da / Num. of mol.: 1 / Source method: obtained synthetically / Details: MIMOTOPE OF THE NICOTINIC ACETYLCHOLINE RECEPTOR / Source: (synth.) SYNTHETIC CONSTRUCT (others) |
| Compound details | THE PEPTIDE BINDS ALPHA-BUNGAROTOXIN AND THUS INHIBITS THE BINDING OF THE TOXIN TO THE NICOTINIC ...THE PEPTIDE BINDS ALPHA-BUNGAROTOX |
| Has protein modification | Y |
-Experimental details
-Experiment
| Experiment | Method: SOLUTION NMR | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| NMR experiment |
| ||||||||||||||||
| NMR details | Text: THE STRUCTURES WERE DETERMINED USING STANDARD 2D 1H-NMR SPECTROSCOPY. |
- Sample preparation
Sample preparation
| Details | Contents: 1MM COMPLEX IN 90% WATER,10% D2O |
|---|---|
| Sample conditions | pH: 6 / Pressure: AMBIENT / Temperature: 303 K |
| Crystal grow | *PLUS Method: other / Details: NMR |
-NMR measurement
| NMR spectrometer |
|
|---|
- Processing
Processing
| NMR software |
| ||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Refinement | Method: DISTANCE GEOMETRY, DYNAMICAL SIMULATED ANNEALING / Software ordinal: 1 Details: RMS DEVIATIONS FROM IDEAL VALUES: BOND LENGTH (A) 0.0033,ANGLES (DEG) 0.52 IMPROPERS (DEG) 0.45 | ||||||||||||||||||||
| NMR ensemble | Conformer selection criteria: NO RESTRAINT VIOLATION / Conformers submitted total number: 10 |
 Movie
Movie Controller
Controller












 PDBj
PDBj

