[English] 日本語
 Yorodumi
Yorodumi- PDB-1f9h: CRYSTAL STRUCTURE OF THE TERNARY COMPLEX OF E. COLI HPPK(R92A) WI... -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 1f9h | ||||||
|---|---|---|---|---|---|---|---|
| Title | CRYSTAL STRUCTURE OF THE TERNARY COMPLEX OF E. COLI HPPK(R92A) WITH MGAMPCPP AND 6-HYDROXYMETHYL-7,8-DIHYDROPTERIN AT 1.50 ANGSTROM RESOLUTION | ||||||
 Components Components | 6-HYDROXYMETHYL-7,8-DIHYDROPTERIN PYROPHOSPHOKINASE | ||||||
 Keywords Keywords | TRANSFERASE / PYROPHOSPHOKINASE / PYROPHOSPHORYL TRANSFER / CATALYTIC MECHANISM / FOLATE / HPPK / PTERIN / 6-HYDROXYMETHYL-7 / 8-DIHYDROPTERIN / TERNARY COMPLEX / SUBSTRATE SPECIFICITY / ANTIMICROBIAL AGENT / DRUG DESIGN | ||||||
| Function / homology |  Function and homology information Function and homology information2-amino-4-hydroxy-6-hydroxymethyldihydropteridine diphosphokinase / 2-amino-4-hydroxy-6-hydroxymethyldihydropteridine diphosphokinase activity / folic acid biosynthetic process / tetrahydrofolate biosynthetic process / kinase activity / magnesium ion binding / ATP binding Similarity search - Function | ||||||
| Biological species |  | ||||||
| Method |  X-RAY DIFFRACTION / X-RAY DIFFRACTION /  SYNCHROTRON / SYNCHROTRON /  MOLECULAR REPLACEMENT / Resolution: 1.5 Å MOLECULAR REPLACEMENT / Resolution: 1.5 Å | ||||||
 Authors Authors | Blaszczyk, J. / Ji, X. | ||||||
 Citation Citation |  Journal: Biochemistry / Year: 2003 Journal: Biochemistry / Year: 2003Title: Dynamic Roles of Arginine Residues 82 and 92 of Escherichia coli 6-Hydroxymethyl-7,8-dihydropterin Pyrophosphokinase: Crystallographic Studies Authors: Blaszczyk, J. / Li, Y. / Shi, G. / Yan, H. / Ji, X. | ||||||
| History |
|
- Structure visualization
Structure visualization
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
|---|
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  1f9h.cif.gz 1f9h.cif.gz | 98.1 KB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb1f9h.ent.gz pdb1f9h.ent.gz | 72 KB | Display |  PDB format PDB format |
| PDBx/mmJSON format |  1f9h.json.gz 1f9h.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/f9/1f9h https://data.pdbj.org/pub/pdb/validation_reports/f9/1f9h ftp://data.pdbj.org/pub/pdb/validation_reports/f9/1f9h ftp://data.pdbj.org/pub/pdb/validation_reports/f9/1f9h | HTTPS FTP |
|---|
-Related structure data
| Related structure data |  1g4cC  1hq2C  1im6C  1kbrC  3ip0C  1eqo S: Starting model for refinement C: citing same article ( |
|---|---|
| Similar structure data |
- Links
Links
- Assembly
Assembly
| Deposited unit | 
| ||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 |
| ||||||||||||||||||||||||
| Unit cell |
| ||||||||||||||||||||||||
| Components on special symmetry positions |
|
- Components
Components
-Protein , 1 types, 1 molecules A
| #1: Protein | Mass: 17880.418 Da / Num. of mol.: 1 / Mutation: R92A Source method: isolated from a genetically manipulated source Source: (gene. exp.)   References: UniProt: P26281, 2-amino-4-hydroxy-6-hydroxymethyldihydropteridine diphosphokinase |
|---|
-Non-polymers , 5 types, 417 molecules 

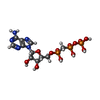






| #2: Chemical | | #3: Chemical | ChemComp-CL / | #4: Chemical | ChemComp-APC / | #5: Chemical | ChemComp-PH2 / | #6: Water | ChemComp-HOH / | |
|---|
-Experimental details
-Experiment
| Experiment | Method:  X-RAY DIFFRACTION / Number of used crystals: 1 X-RAY DIFFRACTION / Number of used crystals: 1 |
|---|
- Sample preparation
Sample preparation
| Crystal | Density Matthews: 1.92 Å3/Da / Density % sol: 33.5 % | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Crystal grow | Temperature: 292 K / Method: vapor diffusion, hanging drop / pH: 5.2 Details: PEG4000, MAGNESIUM CHLORIDE, ACETATE, GLYCEROL, pH 5.20, VAPOR DIFFUSION, HANGING DROP, temperature 292.0K | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Crystal grow | *PLUS Temperature: 18-20 ℃ / pH: 8 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Components of the solutions | *PLUS
|
-Data collection
| Diffraction | Mean temperature: 100 K |
|---|---|
| Diffraction source | Source:  SYNCHROTRON / Site: SYNCHROTRON / Site:  NSLS NSLS  / Beamline: X9B / Wavelength: 0.917 / Beamline: X9B / Wavelength: 0.917 |
| Detector | Type: ADSC QUANTUM 4 / Detector: CCD / Date: Sep 6, 1999 / Details: MIRROR |
| Radiation | Monochromator: SILICON 111 / Protocol: SINGLE WAVELENGTH / Monochromatic (M) / Laue (L): M / Scattering type: x-ray |
| Radiation wavelength | Wavelength: 0.917 Å / Relative weight: 1 |
| Reflection | Resolution: 1.5→30 Å / Num. all: 22905 / Num. obs: 22905 / % possible obs: 99.3 % / Observed criterion σ(F): 0 / Observed criterion σ(I): 0 / Redundancy: 3.418 % / Biso Wilson estimate: 15.7 Å2 / Rmerge(I) obs: 0.1 / Net I/σ(I): 12.1143 |
| Reflection shell | Resolution: 1.5→1.55 Å / Redundancy: 3.05 % / Rmerge(I) obs: 0.449 / Mean I/σ(I) obs: 2.16 / Num. unique all: 2141 / % possible all: 94.2 |
| Reflection | *PLUS Highest resolution: 1.5 Å / Lowest resolution: 30 Å / % possible obs: 93.1 % / Num. measured all: 78292 / Rmerge(I) obs: 0.1 |
| Reflection shell | *PLUS % possible obs: 94.2 % / Mean I/σ(I) obs: 2.2 |
- Processing
Processing
| Software |
| |||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Refinement | Method to determine structure:  MOLECULAR REPLACEMENT MOLECULAR REPLACEMENTStarting model: 1EQO  1eqo Resolution: 1.5→30 Å / Num. parameters: 14342 / Num. restraintsaints: 16916 / Cross valid method: FREE R / σ(F): 0 / σ(I): 0 / Stereochemistry target values: ENGH AND HUBER Details: FULL-MATRIX LEAST-SQUARES PROCEDURE, WITH BLOCK OF PARAMETERS SET FOR EACH CYCLE OF ANISOTROPIC REFINEMENT
| |||||||||||||||||||||||||||||||||
| Solvent computation | Solvent model: MOEWS & KRETSINGER, J. MOL. BIOL. 91(1975) 201-228 | |||||||||||||||||||||||||||||||||
| Refine analyze | Num. disordered residues: 21 / Occupancy sum hydrogen: 1273 / Occupancy sum non hydrogen: 1705 | |||||||||||||||||||||||||||||||||
| Refinement step | Cycle: LAST / Resolution: 1.5→30 Å
| |||||||||||||||||||||||||||||||||
| Refine LS restraints |
| |||||||||||||||||||||||||||||||||
| Software | *PLUS Name: SHELXL / Version: 97 / Classification: refinement | |||||||||||||||||||||||||||||||||
| Refinement | *PLUS Rfactor Rfree: 0.151 / Rfactor Rwork: 0.111 | |||||||||||||||||||||||||||||||||
| Solvent computation | *PLUS | |||||||||||||||||||||||||||||||||
| Displacement parameters | *PLUS | |||||||||||||||||||||||||||||||||
| Refine LS restraints | *PLUS
|
 Movie
Movie Controller
Controller

















 PDBj
PDBj







