+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 1f97 | ||||||
|---|---|---|---|---|---|---|---|
| Title | SOLUBLE PART OF THE JUNCTION ADHESION MOLECULE FROM MOUSE | ||||||
 Components Components | JUNCTION ADHESION MOLECULE | ||||||
 Keywords Keywords | CELL ADHESION / immunoglobulin superfamily / beta-sandwich fold | ||||||
| Function / homology |  Function and homology information Function and homology informationTight junction interactions / positive regulation of establishment of endothelial barrier / TGF-beta receptor signaling in EMT (epithelial to mesenchymal transition) / memory T cell extravasation / Integrin cell surface interactions / establishment of endothelial intestinal barrier / Cell surface interactions at the vascular wall / regulation of membrane permeability / protein localization to bicellular tight junction / negative regulation of stress fiber assembly ...Tight junction interactions / positive regulation of establishment of endothelial barrier / TGF-beta receptor signaling in EMT (epithelial to mesenchymal transition) / memory T cell extravasation / Integrin cell surface interactions / establishment of endothelial intestinal barrier / Cell surface interactions at the vascular wall / regulation of membrane permeability / protein localization to bicellular tight junction / negative regulation of stress fiber assembly / positive regulation of platelet aggregation / actomyosin structure organization / intestinal absorption / regulation of bicellular tight junction assembly / leukocyte cell-cell adhesion / positive regulation of Rho protein signal transduction / bicellular tight junction / epithelial cell differentiation / regulation of cytokine production / protein localization to plasma membrane / PDZ domain binding / cellular response to mechanical stimulus / integrin binding / regulation of cell shape / cell adhesion / protein homodimerization activity / protein-containing complex / plasma membrane Similarity search - Function | ||||||
| Biological species |  | ||||||
| Method |  X-RAY DIFFRACTION / Resolution: 2.5 Å X-RAY DIFFRACTION / Resolution: 2.5 Å | ||||||
 Authors Authors | Kostrewa, D. / Brockhaus, M. / D'Arcy, A. / Dale, G. / Bazzoni, G. / Dejana, E. / Winkler, F. / Hennig, M. | ||||||
 Citation Citation |  Journal: EMBO J. / Year: 2001 Journal: EMBO J. / Year: 2001Title: X-ray structure of junctional adhesion molecule: structural basis for homophilic adhesion via a novel dimerization motif. Authors: Kostrewa, D. / Brockhaus, M. / D'Arcy, A. / Dale, G.E. / Nelboeck, P. / Schmid, G. / Mueller, F. / Bazzoni, G. / Dejana, E. / Bartfai, T. / Winkler, F.K. / Hennig, M. | ||||||
| History |
|
- Structure visualization
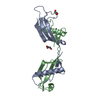
Structure visualization
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
|---|
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  1f97.cif.gz 1f97.cif.gz | 53.5 KB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb1f97.ent.gz pdb1f97.ent.gz | 37.8 KB | Display |  PDB format PDB format |
| PDBx/mmJSON format |  1f97.json.gz 1f97.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/f9/1f97 https://data.pdbj.org/pub/pdb/validation_reports/f9/1f97 ftp://data.pdbj.org/pub/pdb/validation_reports/f9/1f97 ftp://data.pdbj.org/pub/pdb/validation_reports/f9/1f97 | HTTPS FTP |
|---|
-Related structure data
| Similar structure data |
|---|
- Links
Links
- Assembly
Assembly
| Deposited unit | 
| ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 1 |
| ||||||||
| Unit cell |
| ||||||||
| Details | homodimer, under certain conditions a fraction of tetramer |
- Components
Components
| #1: Protein | Mass: 22851.234 Da / Num. of mol.: 1 Source method: isolated from a genetically manipulated source Source: (gene. exp.)   |
|---|---|
| #2: Chemical | ChemComp-MG / |
| #3: Water | ChemComp-HOH / |
| Has protein modification | Y |
-Experimental details
-Experiment
| Experiment | Method:  X-RAY DIFFRACTION / Number of used crystals: 1 X-RAY DIFFRACTION / Number of used crystals: 1 |
|---|
- Sample preparation
Sample preparation
| Crystal | Density Matthews: 2.53 Å3/Da / Density % sol: 51.39 % | ||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Crystal grow | Temperature: 293 K / Method: vapor diffusion, hanging drop / pH: 8.5 Details: 25% Peg 3350, 200 mM MgCl2, 100 mM Tris, pH 8.5, VAPOR DIFFUSION, HANGING DROP, temperature 293K | ||||||||||||||||||||||||||||||
| Crystal grow | *PLUS Method: vapor diffusion | ||||||||||||||||||||||||||||||
| Components of the solutions | *PLUS
|
-Data collection
| Diffraction | Mean temperature: 120 K |
|---|---|
| Diffraction source | Source:  ROTATING ANODE / Type: ELLIOTT GX-21 / Wavelength: 1.5418 ROTATING ANODE / Type: ELLIOTT GX-21 / Wavelength: 1.5418 |
| Detector | Type: SIEMENS HI-STAR / Detector: AREA DETECTOR / Date: Aug 6, 1998 |
| Radiation | Protocol: SINGLE WAVELENGTH / Monochromatic (M) / Laue (L): M / Scattering type: x-ray |
| Radiation wavelength | Wavelength: 1.5418 Å / Relative weight: 1 |
| Reflection | Resolution: 2.5→25 Å / Num. all: 18966 / Num. obs: 18966 / % possible obs: 89.5 % / Observed criterion σ(F): 0 / Observed criterion σ(I): 0 / Redundancy: 2.5 % / Biso Wilson estimate: 24.5 Å2 / Rmerge(I) obs: 0.054 / Net I/σ(I): 14.4 |
| Reflection shell | Resolution: 2.5→2.6 Å / Redundancy: 1.8 % / Rmerge(I) obs: 0.032 / Num. unique all: 7497 / % possible all: 68.3 |
| Reflection | *PLUS Lowest resolution: 25 Å / Num. obs: 7497 / Num. measured all: 18966 |
| Reflection shell | *PLUS % possible obs: 68.3 % / Rmerge(I) obs: 0.13 / Mean I/σ(I) obs: 3.2 |
- Processing
Processing
| Software |
| |||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Refinement | Resolution: 2.5→25 Å / σ(F): 0 / σ(I): 0 / Stereochemistry target values: Engh & Huber
| |||||||||||||||||||||||||
| Refinement step | Cycle: LAST / Resolution: 2.5→25 Å
| |||||||||||||||||||||||||
| Refine LS restraints |
| |||||||||||||||||||||||||
| Software | *PLUS Name:  X-PLOR / Version: 98 / Classification: refinement X-PLOR / Version: 98 / Classification: refinement | |||||||||||||||||||||||||
| Refinement | *PLUS Highest resolution: 2.5 Å / Lowest resolution: 25 Å / σ(F): 0 / % reflection Rfree: 10 % / Rfactor obs: 0.15 / Rfactor Rfree: 0.21 / Rfactor Rwork: 0.15 | |||||||||||||||||||||||||
| Solvent computation | *PLUS | |||||||||||||||||||||||||
| Displacement parameters | *PLUS | |||||||||||||||||||||||||
| Refine LS restraints | *PLUS Type: x_angle_deg / Dev ideal: 1.3 |
 Movie
Movie Controller
Controller












 PDBj
PDBj







