[English] 日本語
 Yorodumi
Yorodumi- PDB-1ear: Crystal structure of Bacillus pasteurii UreE at 1.7 A. Type II cr... -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 1ear | ||||||
|---|---|---|---|---|---|---|---|
| Title | Crystal structure of Bacillus pasteurii UreE at 1.7 A. Type II crystal form. | ||||||
 Components Components | UREASE ACCESSORY PROTEIN UREE | ||||||
 Keywords Keywords | CHAPERONE / PUTATIVE NI-CHAPERONE / UREASE OPERON | ||||||
| Function / homology |  Function and homology information Function and homology informationurea metabolic process / nickel cation binding / unfolded protein binding / protein folding / protein-containing complex assembly / cytoplasm Similarity search - Function | ||||||
| Biological species |  BACILLUS PASTEURII (bacteria) BACILLUS PASTEURII (bacteria) | ||||||
| Method |  X-RAY DIFFRACTION / X-RAY DIFFRACTION /  SYNCHROTRON / SYNCHROTRON /  MOLECULAR REPLACEMENT / Resolution: 1.7 Å MOLECULAR REPLACEMENT / Resolution: 1.7 Å | ||||||
 Authors Authors | Remaut, H. / Safarov, N. / Ciurli, S. / Van Beeumen, J. | ||||||
 Citation Citation |  Journal: J.Biol.Chem. / Year: 2001 Journal: J.Biol.Chem. / Year: 2001Title: Structural Basis for Ni2+ Transport and Assembly of the Urease Active Site by the Metallochaperone Uree from Bacillus Pasteurii Authors: Reamut, H. / Safarov, N. / Ciurli, S. / Van Beeumen, J. | ||||||
| History |
|
- Structure visualization
Structure visualization
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
|---|
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  1ear.cif.gz 1ear.cif.gz | 47 KB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb1ear.ent.gz pdb1ear.ent.gz | 32.5 KB | Display |  PDB format PDB format |
| PDBx/mmJSON format |  1ear.json.gz 1ear.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/ea/1ear https://data.pdbj.org/pub/pdb/validation_reports/ea/1ear ftp://data.pdbj.org/pub/pdb/validation_reports/ea/1ear ftp://data.pdbj.org/pub/pdb/validation_reports/ea/1ear | HTTPS FTP |
|---|
-Related structure data
| Related structure data |  1eb0SC S: Starting model for refinement C: citing same article ( |
|---|---|
| Similar structure data |
- Links
Links
- Assembly
Assembly
| Deposited unit | 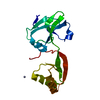
| ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 1 | 
| ||||||||
| Unit cell |
| ||||||||
| Components on special symmetry positions |
|
- Components
Components
| #1: Protein | Mass: 17412.934 Da / Num. of mol.: 1 / Mutation: YES Source method: isolated from a genetically manipulated source Details: PUTATIVE NI-CHAPERONE / Source: (gene. exp.)  BACILLUS PASTEURII (bacteria) / Production host: BACILLUS PASTEURII (bacteria) / Production host:  |
|---|---|
| #2: Chemical | ChemComp-ZN / |
| #3: Water | ChemComp-HOH / |
-Experimental details
-Experiment
| Experiment | Method:  X-RAY DIFFRACTION / Number of used crystals: 1 X-RAY DIFFRACTION / Number of used crystals: 1 |
|---|
- Sample preparation
Sample preparation
| Crystal | Density Matthews: 2.61 Å3/Da / Density % sol: 52.92 % | ||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Crystal grow | Temperature: 294 K / pH: 7 / Details: 92-96 % NACITRATE, 100MM TRIS PH 7, 294 K | ||||||||||||||||||||||||||||||||||||||||||
| Crystal grow | *PLUS Temperature: 294 K / pH: 7.5 / Method: vapor diffusion, hanging drop | ||||||||||||||||||||||||||||||||||||||||||
| Components of the solutions | *PLUS
|
-Data collection
| Diffraction | Mean temperature: 100 K |
|---|---|
| Diffraction source | Source:  SYNCHROTRON / Site: SYNCHROTRON / Site:  EMBL/DESY, HAMBURG EMBL/DESY, HAMBURG  / Beamline: BW7B / Wavelength: 0.8453 / Beamline: BW7B / Wavelength: 0.8453 |
| Detector | Type: MAR scanner 345 mm plate / Detector: IMAGE PLATE / Date: May 15, 2001 |
| Radiation | Protocol: SINGLE WAVELENGTH / Monochromatic (M) / Laue (L): M / Scattering type: x-ray |
| Radiation wavelength | Wavelength: 0.8453 Å / Relative weight: 1 |
| Reflection | Resolution: 1.7→15 Å / Num. obs: 20619 / % possible obs: 99 % / Redundancy: 6.2 % / Biso Wilson estimate: 18.9 Å2 / Rmerge(I) obs: 0.058 / Net I/σ(I): 32.6 |
| Reflection shell | Resolution: 1.7→1.76 Å / Redundancy: 3.2 % / Rmerge(I) obs: 0.281 / Mean I/σ(I) obs: 3.63 / % possible all: 96.8 |
| Reflection | *PLUS Num. measured all: 292340 |
| Reflection shell | *PLUS % possible obs: 96.8 % |
- Processing
Processing
| Software |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Refinement | Method to determine structure:  MOLECULAR REPLACEMENT MOLECULAR REPLACEMENTStarting model: PDB IDCODE 1EB0 Resolution: 1.7→14.85 Å / Rfactor Rfree error: 0.007 / Isotropic thermal model: RESTRAINED / Cross valid method: THROUGHOUT / σ(F): 1.7 Details: B-FACTOR WAS FIXED AT 100 A**2 FOR DISORDERED SIDECHAINS
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Solvent computation | Solvent model: FLAT MODEL / Bsol: 57.8566 Å2 / ksol: 0.416487 e/Å3 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Displacement parameters | Biso mean: 33.7 Å2
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refine analyze |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement step | Cycle: LAST / Resolution: 1.7→14.85 Å
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refine LS restraints |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| LS refinement shell | Resolution: 1.7→1.81 Å / Rfactor Rfree error: 0.029 / Total num. of bins used: 6
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Xplor file |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Software | *PLUS Name: CNS / Version: 1 / Classification: refinement | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement | *PLUS Rfactor obs: 0.2126 / Rfactor Rfree: 0.2281 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Solvent computation | *PLUS | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Displacement parameters | *PLUS | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refine LS restraints | *PLUS
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| LS refinement shell | *PLUS Rfactor obs: 0.305 |
 Movie
Movie Controller
Controller












 PDBj
PDBj



