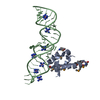
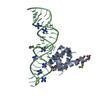
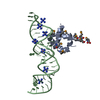
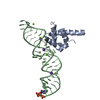
Entry Database : PDB / ID : 1dulTitle STRUCTURE OF THE RIBONUCLEOPROTEIN CORE OF THE E. COLI SIGNAL RECOGNITION PARTICLE 4.5 S RNA DOMAIN IV Signal recognition particle protein Keywords / / / / / / / / Function / homology Function Domain/homology Component
/ / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / Biological species Escherichia coli (E. coli)synthetic construct (others) Method / / Resolution : 1.8 Å Authors Batey, R.T. / Rambo, R.P. / Lucast, L. / Rha, B. / Doudna, J.A. Journal : Science / Year : 2000Title : Crystal structure of the ribonucleoprotein core of the signal recognition particle.Authors : Batey, R.T. / Rambo, R.P. / Lucast, L. / Rha, B. / Doudna, J.A. History Deposition Jan 17, 2000 Deposition site / Processing site Revision 1.0 Feb 28, 2000 Provider / Type Revision 1.1 Apr 27, 2008 Group Revision 1.2 Jul 13, 2011 Group Revision 2.0 Nov 6, 2019 Group Advisory / Atomic model ... Advisory / Atomic model / Data collection / Database references / Derived calculations / Experimental preparation / Non-polymer description / Polymer sequence / Source and taxonomy / Structure summary Category atom_site / chem_comp ... atom_site / chem_comp / entity / entity_name_com / entity_poly / entity_poly_seq / entity_src_gen / exptl_crystal / ndb_struct_na_base_pair / ndb_struct_na_base_pair_step / pdbx_entity_src_syn / pdbx_nonpoly_scheme / pdbx_poly_seq_scheme / pdbx_struct_conn_angle / pdbx_struct_mod_residue / pdbx_unobs_or_zero_occ_atoms / pdbx_unobs_or_zero_occ_residues / pdbx_validate_polymer_linkage / struct_conf / struct_conn / struct_ref / struct_ref_seq / struct_ref_seq_dif / struct_site_gen Item _chem_comp.formula / _chem_comp.formula_weight ... _chem_comp.formula / _chem_comp.formula_weight / _chem_comp.id / _chem_comp.mon_nstd_flag / _chem_comp.name / _chem_comp.type / _entity.formula_weight / _entity.pdbx_description / _entity.pdbx_fragment / _entity_name_com.name / _entity_poly.pdbx_seq_one_letter_code / _entity_poly.pdbx_seq_one_letter_code_can / _entity_src_gen.pdbx_beg_seq_num / _entity_src_gen.pdbx_end_seq_num / _entity_src_gen.pdbx_gene_src_gene / _entity_src_gen.pdbx_seq_type / _exptl_crystal.density_Matthews / _ndb_struct_na_base_pair.hbond_type_12 / _ndb_struct_na_base_pair.hbond_type_28 / _ndb_struct_na_base_pair.propeller / _ndb_struct_na_base_pair_step.roll / _ndb_struct_na_base_pair_step.twist / _pdbx_nonpoly_scheme.asym_id / _pdbx_nonpoly_scheme.auth_seq_num / _pdbx_nonpoly_scheme.ndb_seq_num / _pdbx_nonpoly_scheme.pdb_seq_num / _pdbx_nonpoly_scheme.pdb_strand_id / _pdbx_struct_conn_angle.ptnr1_auth_seq_id / _pdbx_struct_conn_angle.ptnr1_label_seq_id / _pdbx_struct_conn_angle.ptnr2_auth_seq_id / _pdbx_struct_conn_angle.ptnr3_auth_seq_id / _pdbx_struct_conn_angle.ptnr3_label_seq_id / _pdbx_unobs_or_zero_occ_atoms.label_seq_id / _struct_conf.beg_label_seq_id / _struct_conf.end_label_seq_id / _struct_ref.db_code / _struct_ref.db_name / _struct_ref.entity_id / _struct_ref.pdbx_align_begin / _struct_ref.pdbx_db_accession / _struct_ref.pdbx_seq_one_letter_code / _struct_ref_seq.db_align_beg / _struct_ref_seq.db_align_end / _struct_ref_seq.pdbx_auth_seq_align_beg / _struct_ref_seq.pdbx_auth_seq_align_end / _struct_ref_seq.pdbx_db_accession / _struct_ref_seq.pdbx_strand_id / _struct_ref_seq.seq_align_beg / _struct_ref_seq.seq_align_end / _struct_site_gen.auth_seq_id / _struct_site_gen.label_seq_id Revision 2.1 Oct 14, 2020 Group / Structure summaryCategory audit_author / pdbx_struct_conn_angle ... audit_author / pdbx_struct_conn_angle / struct_conn / struct_site Item _audit_author.name / _pdbx_struct_conn_angle.ptnr1_auth_asym_id ... _audit_author.name / _pdbx_struct_conn_angle.ptnr1_auth_asym_id / _pdbx_struct_conn_angle.ptnr1_auth_comp_id / _pdbx_struct_conn_angle.ptnr1_auth_seq_id / _pdbx_struct_conn_angle.ptnr1_label_asym_id / _pdbx_struct_conn_angle.ptnr1_label_atom_id / _pdbx_struct_conn_angle.ptnr1_label_comp_id / _pdbx_struct_conn_angle.ptnr1_label_seq_id / _pdbx_struct_conn_angle.ptnr2_auth_comp_id / _pdbx_struct_conn_angle.ptnr2_auth_seq_id / _pdbx_struct_conn_angle.ptnr2_label_asym_id / _pdbx_struct_conn_angle.ptnr2_label_atom_id / _pdbx_struct_conn_angle.ptnr2_label_comp_id / _pdbx_struct_conn_angle.ptnr3_auth_asym_id / _pdbx_struct_conn_angle.ptnr3_auth_comp_id / _pdbx_struct_conn_angle.ptnr3_auth_seq_id / _pdbx_struct_conn_angle.ptnr3_label_asym_id / _pdbx_struct_conn_angle.ptnr3_label_atom_id / _pdbx_struct_conn_angle.ptnr3_label_comp_id / _pdbx_struct_conn_angle.ptnr3_label_seq_id / _pdbx_struct_conn_angle.value / _struct_conn.pdbx_dist_value / _struct_conn.ptnr1_auth_asym_id / _struct_conn.ptnr1_auth_comp_id / _struct_conn.ptnr1_auth_seq_id / _struct_conn.ptnr1_label_asym_id / _struct_conn.ptnr1_label_atom_id / _struct_conn.ptnr1_label_comp_id / _struct_conn.ptnr1_label_seq_id / _struct_conn.ptnr2_auth_asym_id / _struct_conn.ptnr2_auth_comp_id / _struct_conn.ptnr2_auth_seq_id / _struct_conn.ptnr2_label_asym_id / _struct_conn.ptnr2_label_atom_id / _struct_conn.ptnr2_label_comp_id / _struct_conn.ptnr2_label_seq_id / _struct_site.pdbx_auth_asym_id / _struct_site.pdbx_auth_comp_id / _struct_site.pdbx_auth_seq_id Revision 2.2 Nov 20, 2024 Group / Database references / Structure summaryCategory chem_comp_atom / chem_comp_bond ... chem_comp_atom / chem_comp_bond / database_2 / pdbx_entry_details / pdbx_modification_feature Item / _database_2.pdbx_database_accession
Show all Show less
 Yorodumi
Yorodumi Open data
Open data Basic information
Basic information Components
Components Keywords
Keywords Function and homology information
Function and homology information
 X-RAY DIFFRACTION /
X-RAY DIFFRACTION /  SYNCHROTRON / Resolution: 1.8 Å
SYNCHROTRON / Resolution: 1.8 Å  Authors
Authors Citation
Citation Journal: Science / Year: 2000
Journal: Science / Year: 2000 Structure visualization
Structure visualization Molmil
Molmil Jmol/JSmol
Jmol/JSmol Downloads & links
Downloads & links Download
Download 1dul.cif.gz
1dul.cif.gz PDBx/mmCIF format
PDBx/mmCIF format pdb1dul.ent.gz
pdb1dul.ent.gz PDB format
PDB format 1dul.json.gz
1dul.json.gz PDBx/mmJSON format
PDBx/mmJSON format Other downloads
Other downloads https://data.pdbj.org/pub/pdb/validation_reports/du/1dul
https://data.pdbj.org/pub/pdb/validation_reports/du/1dul ftp://data.pdbj.org/pub/pdb/validation_reports/du/1dul
ftp://data.pdbj.org/pub/pdb/validation_reports/du/1dul Links
Links Assembly
Assembly
 Components
Components

 X-RAY DIFFRACTION / Number of used crystals: 1
X-RAY DIFFRACTION / Number of used crystals: 1  Sample preparation
Sample preparation SYNCHROTRON / Site:
SYNCHROTRON / Site:  NSLS
NSLS  / Beamline: X4A / Wavelength: 0.9793,0.9787,0.9667
/ Beamline: X4A / Wavelength: 0.9793,0.9787,0.9667 Processing
Processing Movie
Movie Controller
Controller








 PDBj
PDBj


































