[English] 日本語
 Yorodumi
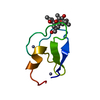
Yorodumi- PDB-1dgz: RIBOSMAL PROTEIN L36 FROM THERMUS THERMOPHILUS: NMR STRUCTURE ENSEMBLE -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 1dgz | ||||||
|---|---|---|---|---|---|---|---|
| Title | RIBOSMAL PROTEIN L36 FROM THERMUS THERMOPHILUS: NMR STRUCTURE ENSEMBLE | ||||||
 Components Components | PROTEIN (L36 RIBOSOMAL PROTEIN) | ||||||
 Keywords Keywords | RIBOSOME / RIBOSOMAL PROTEIN / LARGE RIBOSOMAL SUBUNIT / ZINC BINDING / BETA SHEET | ||||||
| Function / homology |  Function and homology information Function and homology informationstructural constituent of ribosome / ribosome / translation / ribonucleoprotein complex / metal ion binding / cytoplasm Similarity search - Function | ||||||
| Biological species |   Thermus thermophilus (bacteria) Thermus thermophilus (bacteria) | ||||||
| Method | SOLUTION NMR | ||||||
 Authors Authors | Hard, T. / Rak, A. / Allard, P. / Kloo, L. / Garber, M. | ||||||
 Citation Citation |  Journal: J.Mol.Biol. / Year: 2000 Journal: J.Mol.Biol. / Year: 2000Title: The solution structure of ribosomal protein L36 from Thermus thermophilus reveals a zinc-ribbon-like fold. Authors: Hard, T. / Rak, A. / Allard, P. / Kloo, L. / Garber, M. | ||||||
| History |
|
- Structure visualization
Structure visualization
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
|---|
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  1dgz.cif.gz 1dgz.cif.gz | 480 KB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb1dgz.ent.gz pdb1dgz.ent.gz | 399.5 KB | Display |  PDB format PDB format |
| PDBx/mmJSON format |  1dgz.json.gz 1dgz.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/dg/1dgz https://data.pdbj.org/pub/pdb/validation_reports/dg/1dgz ftp://data.pdbj.org/pub/pdb/validation_reports/dg/1dgz ftp://data.pdbj.org/pub/pdb/validation_reports/dg/1dgz | HTTPS FTP |
|---|
-Related structure data
- Links
Links
- Assembly
Assembly
| Deposited unit | 
| |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 1 |
| |||||||||
| NMR ensembles |
|
- Components
Components
| #1: Protein/peptide | Mass: 4435.411 Da / Num. of mol.: 1 Source method: isolated from a genetically manipulated source Source: (gene. exp.)   Thermus thermophilus (bacteria) / Production host: Thermus thermophilus (bacteria) / Production host:  |
|---|---|
| #2: Chemical | ChemComp-ZN / |
-Experimental details
-Experiment
| Experiment | Method: SOLUTION NMR |
|---|
- Sample preparation
Sample preparation
| Crystal grow | *PLUS Method: other / Details: NMR |
|---|
- Processing
Processing
| NMR ensemble | Conformer selection criteria: STRUCTURES WITH ACCEPTABLE COVALENT GEOMETRY,STRUCTURES WITH FAVORABLE NON- BOND ENERGY,STRUCTURES WITH THE LEAST RESTRAINT VIOLATIONS,STRUCTURES WITH THE LOWEST ENERGY Conformers calculated total number: 50 / Conformers submitted total number: 38 |
|---|
 Movie
Movie Controller
Controller








 PDBj
PDBj


