+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 1d2u | ||||||
|---|---|---|---|---|---|---|---|
| Title | 1.15 A CRYSTAL STRUCTURE OF NITROPHORIN 4 FROM RHODNIUS PROLIXUS | ||||||
 Components Components | NITROPHORIN 4 | ||||||
 Keywords Keywords | TRANSPORT PROTEIN / NITRIC OXIDE TRANSPORT / FERRIC HEME / ANTIHISTAMINE / VASODILATOR / LIPOCALIN / BILAN BINDING PROTEIN | ||||||
| Function / homology |  Function and homology information Function and homology informationnitrite dismutase / histamine binding / nitric oxide binding / vasodilation / oxidoreductase activity / extracellular region / metal ion binding Similarity search - Function | ||||||
| Biological species |  | ||||||
| Method |  X-RAY DIFFRACTION / X-RAY DIFFRACTION /  SYNCHROTRON / Resolution: 1.15 Å SYNCHROTRON / Resolution: 1.15 Å | ||||||
 Authors Authors | Weichsel, A. / Andersen, J.F. / Roberts, S.A. / Montfort, W.R. | ||||||
 Citation Citation |  Journal: Biochemistry / Year: 2001 Journal: Biochemistry / Year: 2001Title: Ligand-induced heme ruffling and bent no geometry in ultra-high-resolution structures of nitrophorin 4. Authors: Roberts, S.A. / Weichsel, A. / Qiu, Y. / Shelnutt, J.A. / Walker, F.A. / Montfort, W.R. | ||||||
| History |
|
- Structure visualization
Structure visualization
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
|---|
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  1d2u.cif.gz 1d2u.cif.gz | 93.1 KB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb1d2u.ent.gz pdb1d2u.ent.gz | 70.1 KB | Display |  PDB format PDB format |
| PDBx/mmJSON format |  1d2u.json.gz 1d2u.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Summary document |  1d2u_validation.pdf.gz 1d2u_validation.pdf.gz | 1.1 MB | Display |  wwPDB validaton report wwPDB validaton report |
|---|---|---|---|---|
| Full document |  1d2u_full_validation.pdf.gz 1d2u_full_validation.pdf.gz | 1.1 MB | Display | |
| Data in XML |  1d2u_validation.xml.gz 1d2u_validation.xml.gz | 13.4 KB | Display | |
| Data in CIF |  1d2u_validation.cif.gz 1d2u_validation.cif.gz | 19.8 KB | Display | |
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/d2/1d2u https://data.pdbj.org/pub/pdb/validation_reports/d2/1d2u ftp://data.pdbj.org/pub/pdb/validation_reports/d2/1d2u ftp://data.pdbj.org/pub/pdb/validation_reports/d2/1d2u | HTTPS FTP |
-Related structure data
- Links
Links
- Assembly
Assembly
| Deposited unit | 
| |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 |
| |||||||||||||||
| Unit cell |
| |||||||||||||||
| Components on special symmetry positions |
|
- Components
Components
| #1: Protein | Mass: 20292.664 Da / Num. of mol.: 1 / Fragment: RESIDUES 22-205 Source method: isolated from a genetically manipulated source Source: (gene. exp.)   |
|---|---|
| #2: Chemical | ChemComp-HEM / |
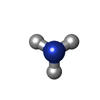
| #3: Chemical | ChemComp-NH3 / |
| #4: Water | ChemComp-HOH / |
| Has protein modification | Y |
| Nonpolymer details | Ammonia (NH3) occupies the Fe sixth coordination position The heme is disordered by a rotation of ...Ammonia (NH3) occupies the Fe sixth coordination position The heme is disordered by a rotation of 180 degrees around the CHA-Fe-CHC axis. The only evidence of this disorder is the appearence of methyl groups CMB and CMC as vinyls. These extra atoms are called CBBB and CBCB. THE LOOP CONTAINING |
-Experimental details
-Experiment
| Experiment | Method:  X-RAY DIFFRACTION / Number of used crystals: 1 X-RAY DIFFRACTION / Number of used crystals: 1 |
|---|
- Sample preparation
Sample preparation
| Crystal | Density Matthews: 1.94 Å3/Da / Density % sol: 36.44 % | |||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Crystal grow | Temperature: 298 K / Method: vapor diffusion, hanging drop / pH: 7.5 Details: 3.0 M AMMONIUM PHOSPHATE 10 MM TRIS-HCL, pH 7.5, VAPOR DIFFUSION, HANGING DROP, temperature 298K | |||||||||||||||||||||||||
| Crystal grow | *PLUS pH: 5.6 / Details: Weichsel, A., (2000) Nat. Struct. Biol., 7, 551. | |||||||||||||||||||||||||
| Components of the solutions | *PLUS
|
-Data collection
| Diffraction | Mean temperature: 105 K |
|---|---|
| Diffraction source | Source:  SYNCHROTRON / Site: SYNCHROTRON / Site:  ESRF ESRF  / Beamline: BM14 / Wavelength: 0.8727 / Beamline: BM14 / Wavelength: 0.8727 |
| Detector | Type: MARRESEARCH / Detector: AREA DETECTOR / Date: Dec 11, 1998 |
| Radiation | Protocol: SINGLE WAVELENGTH / Monochromatic (M) / Laue (L): M / Scattering type: x-ray |
| Radiation wavelength | Wavelength: 0.8727 Å / Relative weight: 1 |
| Reflection | Resolution: 1.15→52 Å / Num. all: 49629 / Num. obs: 49629 / % possible obs: 90 % / Observed criterion σ(F): 0 / Observed criterion σ(I): 0 / Redundancy: 2.4 % / Biso Wilson estimate: 10.5 Å2 / Rmerge(I) obs: 0.04 / Net I/σ(I): 22.4 |
| Reflection shell | Resolution: 1.15→1.19 Å / Redundancy: 1.7 % / Rmerge(I) obs: 0.26 / % possible all: 74 |
| Reflection | *PLUS % possible obs: 90 % / Num. measured all: 118648 |
| Reflection shell | *PLUS % possible obs: 74 % |
- Processing
Processing
| Software |
| |||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Refinement | Resolution: 1.15→52 Å / Num. parameters: 15661 / Num. restraintsaints: 18721 / σ(F): 0 / σ(I): 0 / Stereochemistry target values: ENGH & HUBER Details: SHELXL97 REFINEMENT. FE-HEME AND FE-LIGAND DISTANCES AND ANGLES WERE UNRESTRAINED.
| |||||||||||||||||||||||||||||||||
| Refine analyze | Num. disordered residues: 6 | |||||||||||||||||||||||||||||||||
| Refinement step | Cycle: LAST / Resolution: 1.15→52 Å
| |||||||||||||||||||||||||||||||||
| Refine LS restraints |
| |||||||||||||||||||||||||||||||||
| Software | *PLUS Name: SHELXL-97 / Classification: refinement | |||||||||||||||||||||||||||||||||
| Refinement | *PLUS Lowest resolution: 52 Å / σ(F): 0 / % reflection Rfree: 5 % / Rfactor all: 0.13 / Rfactor Rfree: 0.19 / Rfactor Rwork: 0.13 | |||||||||||||||||||||||||||||||||
| Solvent computation | *PLUS | |||||||||||||||||||||||||||||||||
| Displacement parameters | *PLUS | |||||||||||||||||||||||||||||||||
| Refine LS restraints | *PLUS
|
 Movie
Movie Controller
Controller
















 PDBj
PDBj