[English] 日本語
 Yorodumi
Yorodumi- PDB-1bgq: RADICICOL BOUND TO THE ATP BINDING SITE OF THE N-TERMINAL DOMAIN ... -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 1bgq | ||||||
|---|---|---|---|---|---|---|---|
| Title | RADICICOL BOUND TO THE ATP BINDING SITE OF THE N-TERMINAL DOMAIN OF THE YEAST HSP90 CHAPERONE | ||||||
 Components Components | HEAT SHOCK PROTEIN 90 | ||||||
 Keywords Keywords | CHAPERONE / ATP-BINDING / HEAT SHOCK / INHIBITOR | ||||||
| Function / homology |  Function and homology information Function and homology informationThe NLRP3 inflammasome / Tetrahydrobiopterin (BH4) synthesis, recycling, salvage and regulation / eNOS activation / Extra-nuclear estrogen signaling / VEGFR2 mediated vascular permeability / HSF1-dependent transactivation / HSP90 chaperone cycle for steroid hormone receptors (SHR) in the presence of ligand / HSF1 activation / response to oxygen levels / response to osmotic stress ...The NLRP3 inflammasome / Tetrahydrobiopterin (BH4) synthesis, recycling, salvage and regulation / eNOS activation / Extra-nuclear estrogen signaling / VEGFR2 mediated vascular permeability / HSF1-dependent transactivation / HSP90 chaperone cycle for steroid hormone receptors (SHR) in the presence of ligand / HSF1 activation / response to oxygen levels / response to osmotic stress / box C/D snoRNP assembly / regulation of telomere maintenance / 'de novo' protein folding / : / proteasome assembly / positive regulation of telomere maintenance via telomerase / Neutrophil degranulation / protein maturation / ATP-dependent protein folding chaperone / unfolded protein binding / protein folding / cellular response to heat / protein refolding / protein stabilization / perinuclear region of cytoplasm / ATP hydrolysis activity / protein-containing complex / ATP binding / identical protein binding / nucleus / plasma membrane / cytosol / cytoplasm Similarity search - Function | ||||||
| Biological species |  | ||||||
| Method |  X-RAY DIFFRACTION / ISOMORPHOUS REPLACEMENT / Resolution: 2.5 Å X-RAY DIFFRACTION / ISOMORPHOUS REPLACEMENT / Resolution: 2.5 Å | ||||||
 Authors Authors | Roe, S.M. / Prodromou, C. / Pearl, L.H. | ||||||
 Citation Citation |  Journal: J.Med.Chem. / Year: 1999 Journal: J.Med.Chem. / Year: 1999Title: Structural basis for inhibition of the Hsp90 molecular chaperone by the antitumor antibiotics radicicol and geldanamycin. Authors: Roe, S.M. / Prodromou, C. / O'Brien, R. / Ladbury, J.E. / Piper, P.W. / Pearl, L.H. #1:  Journal: Cell(Cambridge,Mass.) / Year: 1997 Journal: Cell(Cambridge,Mass.) / Year: 1997Title: Identification and Structural Characterization of the ATP/Adp-Binding Site in the Hsp90 Molecular Chaperone Authors: Prodromou, C. / Roe, S.M. / O'Brien, R. / Ladbury, J.E. / Piper, P.W. / Pearl, L.H. #2:  Journal: Proteins / Year: 1996 Journal: Proteins / Year: 1996Title: Expression and Crystallization of the Yeast Hsp82 Chaperone, and Preliminary X-Ray Diffraction Studies of the Amino-Terminal Domain Authors: Prodromou, C. / Piper, P.W. / Pearl, L.H. | ||||||
| History |
|
- Structure visualization
Structure visualization
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
|---|
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  1bgq.cif.gz 1bgq.cif.gz | 58.1 KB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb1bgq.ent.gz pdb1bgq.ent.gz | 41.3 KB | Display |  PDB format PDB format |
| PDBx/mmJSON format |  1bgq.json.gz 1bgq.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/bg/1bgq https://data.pdbj.org/pub/pdb/validation_reports/bg/1bgq ftp://data.pdbj.org/pub/pdb/validation_reports/bg/1bgq ftp://data.pdbj.org/pub/pdb/validation_reports/bg/1bgq | HTTPS FTP |
|---|
-Related structure data
| Related structure data |  1ah6S S: Starting model for refinement |
|---|---|
| Similar structure data |
- Links
Links
- Assembly
Assembly
| Deposited unit | 
| ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 1 | 
| ||||||||
| Unit cell |
|
- Components
Components
| #1: Protein | Mass: 25527.037 Da / Num. of mol.: 1 / Fragment: N-TERMINAL DOMAIN Source method: isolated from a genetically manipulated source Source: (gene. exp.)  Cell line: BL21 / Plasmid: PRSETA / Production host:  |
|---|---|
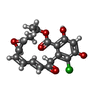
| #2: Chemical | ChemComp-RDC / |
| #3: Water | ChemComp-HOH / |
-Experimental details
-Experiment
| Experiment | Method:  X-RAY DIFFRACTION / Number of used crystals: 1 X-RAY DIFFRACTION / Number of used crystals: 1 |
|---|
- Sample preparation
Sample preparation
| Crystal | Density Matthews: 2.97 Å3/Da / Density % sol: 60 % | ||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Crystal grow | pH: 5 / Details: pH 5.0 | ||||||||||||||||||||||||||||||
| Crystal grow | *PLUS Temperature: 16 ℃ / Method: batch method | ||||||||||||||||||||||||||||||
| Components of the solutions | *PLUS
|
-Data collection
| Diffraction | Mean temperature: 120 K |
|---|---|
| Diffraction source | Source:  ROTATING ANODE / Type: RIGAKU RUH2R / Wavelength: 1.5418 ROTATING ANODE / Type: RIGAKU RUH2R / Wavelength: 1.5418 |
| Detector | Type: MARRESEARCH / Detector: IMAGE PLATE / Date: Feb 1, 1997 / Details: MSC MIRRORS |
| Radiation | Monochromatic (M) / Laue (L): M / Scattering type: x-ray |
| Radiation wavelength | Wavelength: 1.5418 Å / Relative weight: 1 |
| Reflection | Resolution: 2.5→20 Å / Num. obs: 11672 / % possible obs: 99.1 % / Observed criterion σ(I): 1 / Redundancy: 4.4 % / Biso Wilson estimate: 25.9 Å2 / Rsym value: 0.073 / Net I/σ(I): 8.4 |
| Reflection shell | Resolution: 2.46→2.58 Å / Redundancy: 4.4 % / Mean I/σ(I) obs: 3.1 / Rsym value: 0.233 / % possible all: 99.1 |
| Reflection | *PLUS Rmerge(I) obs: 0.073 |
| Reflection shell | *PLUS % possible obs: 99.1 % / Rmerge(I) obs: 0.233 |
- Processing
Processing
| Software |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Refinement | Method to determine structure: ISOMORPHOUS REPLACEMENT Starting model: 1AH6 Resolution: 2.5→30 Å / Rfactor Rfree error: 0.011 / Data cutoff high absF: 10000000 / Data cutoff low absF: 0.001 / Isotropic thermal model: RESTRAINED / Cross valid method: THROUGHOUT / σ(F): 1 / Details: BULK SOLVENT MODEL USED
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Displacement parameters | Biso mean: 24.1 Å2 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refine analyze |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement step | Cycle: LAST / Resolution: 2.5→30 Å
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refine LS restraints |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| LS refinement shell | Resolution: 2.5→2.66 Å / Rfactor Rfree error: 0.034 / Total num. of bins used: 6
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Xplor file |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Software | *PLUS Name:  X-PLOR / Version: 3.851 / Classification: refinement X-PLOR / Version: 3.851 / Classification: refinement | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement | *PLUS Rfactor obs: 0.193 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Solvent computation | *PLUS | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Displacement parameters | *PLUS | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refine LS restraints | *PLUS
|
 Movie
Movie Controller
Controller












 PDBj
PDBj