[English] 日本語
 Yorodumi
Yorodumi- PDB-1bcs: COMPLEX OF THE WHEAT SERINE CARBOXYPEPTIDASE, CPDW-II, WITH THE M... -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 1bcs | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | COMPLEX OF THE WHEAT SERINE CARBOXYPEPTIDASE, CPDW-II, WITH THE MICROBIAL PEPTIDE ALDEHYDE INHIBITOR, CHYMOSTATIN, AND ARGININE AT 100 DEGREES KELVIN | |||||||||
 Components Components |
| |||||||||
 Keywords Keywords | HYDROLASE/HYDROLASE INHIBITOR / MICROBIAL PEPTIDE ALDEHYDE INHIBITOR / COMPLEX (SERINE PROTEASE-INHIBITOR) COMPLEX / HYDROLASE-HYDROLASE INHIBITOR COMPLEX | |||||||||
| Function / homology |  Function and homology information Function and homology informationcarboxypeptidase D / serine-type carboxypeptidase activity / proteolysis Similarity search - Function | |||||||||
| Biological species |   Streptomyces hygroscopicus (bacteria) Streptomyces hygroscopicus (bacteria)MC521-C8 | |||||||||
| Method |  X-RAY DIFFRACTION / Resolution: 2.08 Å X-RAY DIFFRACTION / Resolution: 2.08 Å | |||||||||
 Authors Authors | Bullock, T.L. / Remington, S.J. | |||||||||
 Citation Citation |  Journal: J.Mol.Biol. / Year: 1996 Journal: J.Mol.Biol. / Year: 1996Title: Peptide aldehyde complexes with wheat serine carboxypeptidase II: implications for the catalytic mechanism and substrate specificity. Authors: Bullock, T.L. / Breddam, K. / Remington, S.J. #1:  Journal: Biochemistry / Year: 1994 Journal: Biochemistry / Year: 1994Title: Structure of the Complex of L-Benzylsuccinate with Wheat Serine Carboxypeptidase at 2.0 Angstrom Resolution Authors: Bullock, T.L. / Branchaud, B. / Remington, S.J. #2:  Journal: Biochemistry / Year: 1992 Journal: Biochemistry / Year: 1992Title: Refined Atomic Model of Wheat Serine Carboxypeptidase II at 2.2 Angstrom Resolution Authors: Liao, D.I. / Breddam, K. / Sweet, R.M. / Bullock, T. / Remington, S.J. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
|---|
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  1bcs.cif.gz 1bcs.cif.gz | 110.6 KB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb1bcs.ent.gz pdb1bcs.ent.gz | 81.5 KB | Display |  PDB format PDB format |
| PDBx/mmJSON format |  1bcs.json.gz 1bcs.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/bc/1bcs https://data.pdbj.org/pub/pdb/validation_reports/bc/1bcs ftp://data.pdbj.org/pub/pdb/validation_reports/bc/1bcs ftp://data.pdbj.org/pub/pdb/validation_reports/bc/1bcs | HTTPS FTP |
|---|
-Related structure data
- Links
Links
- Assembly
Assembly
| Deposited unit | 
| ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 1 | 
| ||||||||
| Unit cell |
| ||||||||
| Atom site foot note | 1: CIS PROLINE - PRO A 43 / 2: CIS PROLINE - PRO A 54 / 3: CIS PROLINE - PRO A 96 |
- Components
Components
-SERINE CARBOXYPEPTIDASE ... , 2 types, 2 molecules AB
| #1: Protein | Mass: 29180.295 Da / Num. of mol.: 1 / Source method: isolated from a natural source / Source: (natural)  |
|---|---|
| #2: Protein | Mass: 17995.229 Da / Num. of mol.: 1 / Source method: isolated from a natural source / Source: (natural)  |
-Protein/peptide , 1 types, 1 molecules C
| #3: Protein/peptide | |
|---|
-Sugars , 3 types, 3 molecules 
| #4: Polysaccharide | alpha-L-fucopyranose-(1-3)-[2-acetamido-2-deoxy-alpha-D-glucopyranose-(1-4)]2-acetamido-2-deoxy- ...alpha-L-fucopyranose-(1-3)-[2-acetamido-2-deoxy-alpha-D-glucopyranose-(1-4)]2-acetamido-2-deoxy-beta-D-glucopyranose Source method: isolated from a genetically manipulated source |
|---|---|
| #5: Polysaccharide | 2-acetamido-2-deoxy-beta-D-glucopyranose-(1-4)-2-acetamido-2-deoxy-beta-D-glucopyranose Source method: isolated from a genetically manipulated source |
| #7: Sugar | ChemComp-NAG / |
-Non-polymers , 4 types, 387 molecules 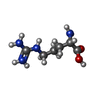






| #6: Chemical | ChemComp-ARG / |
|---|---|
| #8: Chemical | ChemComp-GOL / |
| #9: Chemical | ChemComp-ACT / |
| #10: Water | ChemComp-HOH / |
-Details
| Has protein modification | Y |
|---|
-Experimental details
-Experiment
| Experiment | Method:  X-RAY DIFFRACTION X-RAY DIFFRACTION |
|---|
- Sample preparation
Sample preparation
| Crystal | Density Matthews: 4.94 Å3/Da / Density % sol: 75.11 % | ||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Crystal grow | *PLUS pH: 4 / Method: vapor diffusion | ||||||||||||||||||||||||||||||||||||
| Components of the solutions | *PLUS
|
-Data collection
| Diffraction | Mean temperature: 100 K |
|---|---|
| Diffraction source | Wavelength: 1.54 |
| Detector | Type: RIGAKU / Detector: IMAGE PLATE |
| Radiation | Monochromatic (M) / Laue (L): M / Scattering type: x-ray |
| Radiation wavelength | Wavelength: 1.54 Å / Relative weight: 1 |
| Reflection | Redundancy: 2.75 % / Rmerge(I) obs: 0.04 |
| Reflection | *PLUS Highest resolution: 2.1 Å / Num. obs: 46630 / % possible obs: 81 % / Num. measured all: 127152 / Rmerge(I) obs: 0.04 |
| Reflection shell | *PLUS Highest resolution: 2.1 Å / Lowest resolution: 2.2 Å / % possible obs: 23 % |
- Processing
Processing
| Software |
| ||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Refinement | Resolution: 2.08→33 Å / σ(F): 0 /
| ||||||||||||||||||||||||||||||||||||||||
| Refinement step | Cycle: LAST / Resolution: 2.08→33 Å
| ||||||||||||||||||||||||||||||||||||||||
| Refine LS restraints |
| ||||||||||||||||||||||||||||||||||||||||
| Software | *PLUS Name: TNT / Classification: refinement | ||||||||||||||||||||||||||||||||||||||||
| Refinement | *PLUS Highest resolution: 2.1 Å | ||||||||||||||||||||||||||||||||||||||||
| Solvent computation | *PLUS | ||||||||||||||||||||||||||||||||||||||||
| Displacement parameters | *PLUS | ||||||||||||||||||||||||||||||||||||||||
| Refine LS restraints | *PLUS
|
 Movie
Movie Controller
Controller













 PDBj
PDBj