+ Open data
Open data
- Basic information
Basic information
| Entry | Database: EMDB / ID: EMD-9905 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | RSC substrate-recruitment module | |||||||||
 Map data Map data | RSC SRM region | |||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | chromatin remodeler / SWI/SNF family / DNA BINDING PROTEIN | |||||||||
| Function / homology |  Function and homology information Function and homology informationregulation of sporulation resulting in formation of a cellular spore / : / regulation of nuclear cell cycle DNA replication / plasmid maintenance / DNA translocase activity / nucleosome array spacer activity / : / RSC-type complex / ATP-dependent chromatin remodeler activity / nucleosome disassembly ...regulation of sporulation resulting in formation of a cellular spore / : / regulation of nuclear cell cycle DNA replication / plasmid maintenance / DNA translocase activity / nucleosome array spacer activity / : / RSC-type complex / ATP-dependent chromatin remodeler activity / nucleosome disassembly / UV-damage excision repair / SWI/SNF complex / sister chromatid cohesion / sporulation resulting in formation of a cellular spore / nuclear chromosome / rRNA transcription / histone H4 reader activity / chromosome, centromeric region / nucleosome binding / cytoskeleton organization / meiotic cell cycle / transcription coregulator activity / chromosome segregation / transcription elongation by RNA polymerase II / positive regulation of transcription elongation by RNA polymerase II / helicase activity / double-strand break repair via homologous recombination / base-excision repair / chromatin DNA binding / double-strand break repair via nonhomologous end joining / G2/M transition of mitotic cell cycle / double-strand break repair / histone binding / sequence-specific DNA binding / DNA helicase / DNA-binding transcription factor activity, RNA polymerase II-specific / chromatin remodeling / chromatin binding / regulation of transcription by RNA polymerase II / regulation of DNA-templated transcription / positive regulation of DNA-templated transcription / chromatin / structural molecule activity / positive regulation of transcription by RNA polymerase II / ATP hydrolysis activity / DNA binding / zinc ion binding / ATP binding / nucleus Similarity search - Function | |||||||||
| Biological species |   | |||||||||
| Method | single particle reconstruction / cryo EM / Resolution: 3.4 Å | |||||||||
 Authors Authors | Ye YP / Wu H | |||||||||
 Citation Citation |  Journal: Science / Year: 2019 Journal: Science / Year: 2019Title: Structure of the RSC complex bound to the nucleosome. Authors: Youpi Ye / Hao Wu / Kangjing Chen / Cedric R Clapier / Naveen Verma / Wenhao Zhang / Haiteng Deng / Bradley R Cairns / Ning Gao / Zhucheng Chen /   Abstract: The RSC complex remodels chromatin structure and regulates gene transcription. We used cryo-electron microscopy to determine the structure of yeast RSC bound to the nucleosome. RSC is delineated into ...The RSC complex remodels chromatin structure and regulates gene transcription. We used cryo-electron microscopy to determine the structure of yeast RSC bound to the nucleosome. RSC is delineated into the adenosine triphosphatase motor, the actin-related protein module, and the substrate recruitment module (SRM). RSC binds the nucleosome mainly through the motor, with the auxiliary subunit Sfh1 engaging the H2A-H2B acidic patch to enable nucleosome ejection. SRM is organized into three substrate-binding lobes poised to bind their respective nucleosomal epitopes. The relative orientations of the SRM and the motor on the nucleosome explain the directionality of DNA translocation and promoter nucleosome repositioning by RSC. Our findings shed light on RSC assembly and functionality, and they provide a framework to understand the mammalian homologs BAF/PBAF and the Sfh1 ortholog INI1/BAF47, which are frequently mutated in cancers. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Movie |
 Movie viewer Movie viewer |
|---|---|
| Structure viewer | EM map:  SurfView SurfView Molmil Molmil Jmol/JSmol Jmol/JSmol |
| Supplemental images |
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_9905.map.gz emd_9905.map.gz | 13.4 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-9905-v30.xml emd-9905-v30.xml emd-9905.xml emd-9905.xml | 30.8 KB 30.8 KB | Display Display |  EMDB header EMDB header |
| Images |  emd_9905.png emd_9905.png | 50.5 KB | ||
| Filedesc metadata |  emd-9905.cif.gz emd-9905.cif.gz | 10.2 KB | ||
| Others |  emd_9905_additional_1.map.gz emd_9905_additional_1.map.gz emd_9905_additional_2.map.gz emd_9905_additional_2.map.gz | 166.6 MB 3.1 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-9905 http://ftp.pdbj.org/pub/emdb/structures/EMD-9905 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-9905 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-9905 | HTTPS FTP |
-Related structure data
| Related structure data |  6k15MC  0777C  0778C  6kw3C  6kw4C M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_9905.map.gz / Format: CCP4 / Size: 178 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_9905.map.gz / Format: CCP4 / Size: 178 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | RSC SRM region | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 1.07 Å | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
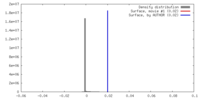
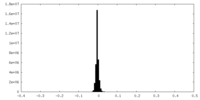
| Density |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
CCP4 map header:
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
-Supplemental data
-Additional map: RSC HB lobe
| File | emd_9905_additional_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | RSC HB lobe | ||||||||||||
| Projections & Slices |
| ||||||||||||
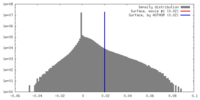
| Density Histograms |
-Additional map: RSC DB lobe
| File | emd_9905_additional_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | RSC DB lobe | ||||||||||||
| Projections & Slices |
| ||||||||||||
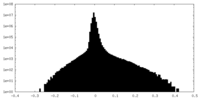
| Density Histograms |
- Sample components
Sample components
+Entire : RSC
+Supramolecule #1: RSC
+Macromolecule #1: Chromatin structure-remodeling complex subunit RSC7
+Macromolecule #2: Chromatin structure-remodeling complex protein RSC8
+Macromolecule #3: Chromatin structure-remodeling complex subunit RSC9
+Macromolecule #4: Chromatin structure-remodeling complex protein RSC6
+Macromolecule #5: Chromatin structure-remodeling complex subunit SFH1
+Macromolecule #6: Chromatin structure-remodeling complex protein RSC58
+Macromolecule #7: Nuclear protein STH1/NPS1
+Macromolecule #8: High temperature lethal protein 1
+Macromolecule #9: Chromatin structure-remodeling complex protein RSC30
+Macromolecule #10: Chromatin structure-remodeling complex protein RSC3
+Macromolecule #11: Chromatin structure-remodeling complex subunit RSC4
+Macromolecule #12: Chromatin structure-remodeling complex subunit RSC2
+Macromolecule #13: ZINC ION
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | cell |
- Sample preparation
Sample preparation
| Buffer | pH: 7.5 |
|---|---|
| Vitrification | Cryogen name: ETHANE |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Image recording | Film or detector model: GATAN K2 QUANTUM (4k x 4k) / Average electron dose: 2.0 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: SPOT SCAN / Imaging mode: DARK FIELD |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
- Image processing
Image processing
| Startup model | Type of model: EMDB MAP |
|---|---|
| Final reconstruction | Resolution.type: BY AUTHOR / Resolution: 3.4 Å / Resolution method: FSC 0.143 CUT-OFF / Number images used: 280000 |
| Initial angle assignment | Type: MAXIMUM LIKELIHOOD |
| Final angle assignment | Type: MAXIMUM LIKELIHOOD |
 Movie
Movie Controller
Controller

















 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)