[English] 日本語
 Yorodumi
Yorodumi- EMDB-9316: Dihedral oligomeric complex of GyrA N-terminal fragment with DNA,... -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: EMDB / ID: EMD-9316 | ||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Title | Dihedral oligomeric complex of GyrA N-terminal fragment with DNA, solved by cryoEM in C2 symmetry | ||||||||||||||||||
 Map data Map data | Dihedral oligomeric complex of GyrA N-terminal fragment with DNA, solved by cryoEM in C2 symmetry | ||||||||||||||||||
 Sample Sample |
| ||||||||||||||||||
 Keywords Keywords | topoisomerase / oligomeric complex / DNA complex / gyrase / T-segment / ISOMERASE-DNA complex | ||||||||||||||||||
| Function / homology |  Function and homology information Function and homology informationDNA topoisomerase type II (double strand cut, ATP-hydrolyzing) complex / DNA negative supercoiling activity / DNA topoisomerase type II (double strand cut, ATP-hydrolyzing) activity / DNA topoisomerase (ATP-hydrolysing) / DNA topological change / DNA-templated DNA replication / chromosome / DNA binding / ATP binding / cytoplasm Similarity search - Function | ||||||||||||||||||
| Biological species |  Streptococcus pneumoniae G54 (bacteria) / Cloning vector pBR322 (others) Streptococcus pneumoniae G54 (bacteria) / Cloning vector pBR322 (others) | ||||||||||||||||||
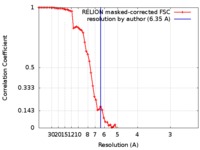
| Method | single particle reconstruction / cryo EM / Resolution: 6.35 Å | ||||||||||||||||||
 Authors Authors | Soczek KM / Grant T | ||||||||||||||||||
| Funding support |  United States, United States,  United Kingdom, 5 items United Kingdom, 5 items
| ||||||||||||||||||
 Citation Citation |  Journal: Elife / Year: 2018 Journal: Elife / Year: 2018Title: CryoEM structures of open dimers of gyrase A in complex with DNA illuminate mechanism of strand passage. Authors: Katarzyna M Soczek / Tim Grant / Peter B Rosenthal / Alfonso Mondragón /   Abstract: Gyrase is a unique type IIA topoisomerase that uses ATP hydrolysis to maintain the negatively supercoiled state of bacterial DNA. In order to perform its function, gyrase undergoes a sequence of ...Gyrase is a unique type IIA topoisomerase that uses ATP hydrolysis to maintain the negatively supercoiled state of bacterial DNA. In order to perform its function, gyrase undergoes a sequence of conformational changes that consist of concerted gate openings, DNA cleavage, and DNA strand passage events. Structures where the transported DNA molecule (T-segment) is trapped by the A subunit have not been observed. Here we present the cryoEM structures of two oligomeric complexes of open gyrase A dimers and DNA. The protein subunits in these complexes were solved to 4 Å and 5.2 Å resolution. One of the complexes traps a linear DNA molecule, a putative T-segment, which interacts with the open gyrase A dimers in two states, representing steps either prior to or after passage through the DNA-gate. The structures locate the T-segment in important intermediate conformations of the catalytic cycle and provide insights into gyrase-DNA interactions and mechanism. | ||||||||||||||||||
| History |
|
- Structure visualization
Structure visualization
| Movie |
 Movie viewer Movie viewer |
|---|---|
| Structure viewer | EM map:  SurfView SurfView Molmil Molmil Jmol/JSmol Jmol/JSmol |
| Supplemental images |
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_9316.map.gz emd_9316.map.gz | 28.5 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-9316-v30.xml emd-9316-v30.xml emd-9316.xml emd-9316.xml | 19.4 KB 19.4 KB | Display Display |  EMDB header EMDB header |
| FSC (resolution estimation) |  emd_9316_fsc.xml emd_9316_fsc.xml | 7.2 KB | Display |  FSC data file FSC data file |
| Images |  emd_9316.png emd_9316.png | 42.4 KB | ||
| Filedesc metadata |  emd-9316.cif.gz emd-9316.cif.gz | 6.9 KB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-9316 http://ftp.pdbj.org/pub/emdb/structures/EMD-9316 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-9316 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-9316 | HTTPS FTP |
-Related structure data
| Related structure data |  6n1pMC  9317C  9318C  6n1qC  6n1rC C: citing same article ( M: atomic model generated by this map |
|---|---|
| Similar structure data |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_9316.map.gz / Format: CCP4 / Size: 30.5 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_9316.map.gz / Format: CCP4 / Size: 30.5 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Dihedral oligomeric complex of GyrA N-terminal fragment with DNA, solved by cryoEM in C2 symmetry | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 1.24 Å | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Density |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
CCP4 map header:
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
-Supplemental data
- Sample components
Sample components
-Entire : Complex of DNA Gyrase A subunit with DNA in C2 symmetry
| Entire | Name: Complex of DNA Gyrase A subunit with DNA in C2 symmetry |
|---|---|
| Components |
|
-Supramolecule #1: Complex of DNA Gyrase A subunit with DNA in C2 symmetry
| Supramolecule | Name: Complex of DNA Gyrase A subunit with DNA in C2 symmetry type: complex / ID: 1 / Parent: 0 / Macromolecule list: all |
|---|---|
| Molecular weight | Theoretical: 490 KDa |
-Supramolecule #2: DNA Gyrase A
| Supramolecule | Name: DNA Gyrase A / type: complex / ID: 2 / Parent: 1 / Macromolecule list: #1 |
|---|---|
| Source (natural) | Organism:  Streptococcus pneumoniae G54 (bacteria) Streptococcus pneumoniae G54 (bacteria) |
-Supramolecule #3: DNA
| Supramolecule | Name: DNA / type: complex / ID: 3 / Parent: 1 / Macromolecule list: #2-#3 |
|---|---|
| Source (natural) | Organism: Cloning vector pBR322 (others) |
-Macromolecule #1: DNA gyrase subunit A
| Macromolecule | Name: DNA gyrase subunit A / type: protein_or_peptide / ID: 1 / Number of copies: 8 / Enantiomer: LEVO / EC number: ec: 5.99.1.3 |
|---|---|
| Source (natural) | Organism:  Streptococcus pneumoniae G54 (bacteria) Streptococcus pneumoniae G54 (bacteria) |
| Molecular weight | Theoretical: 57.977578 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: MHHHHHHSSG VDLGTENLYF QSIAMQDKNL VNVNLTKEMK ASFIDYAMSV IVARALPDVR DGLKPVHRRI LYGMNELGVT PDKPHKKSA RITGDVMGKY HPHGDSSIYE AMVRMAQWWS YRYMLVDGHG NFGSMDGDSA AAQRYTEARM SKIALEMLRD I NKNTVDFV ...String: MHHHHHHSSG VDLGTENLYF QSIAMQDKNL VNVNLTKEMK ASFIDYAMSV IVARALPDVR DGLKPVHRRI LYGMNELGVT PDKPHKKSA RITGDVMGKY HPHGDSSIYE AMVRMAQWWS YRYMLVDGHG NFGSMDGDSA AAQRYTEARM SKIALEMLRD I NKNTVDFV DNYDANEREP LVLPARFPNL LVNGATGIAV GMATNIPPHN LGETIDAVKL VMDNPEVTTK DLMEVLPGPD FP TGALVMG KSGIHKAYET GKGSIVLRSR TEIETTKTGR ERIVVTEFPY MVNKTKVHEH IVRLVQEKRI EGITAVRDES NRE GVRFVI EVKRDASANV ILNNLFKMTQ MQTNFGFNML AIQNGIPKIL SLRQILDAYI EHQKEVVVRR TRFDKEKAEA RAHI LEGLL IALDHIDEVI RIIRASETDA EAQAELMSKF KLSERQSQAI LDMRLRRLTG LERDKIQSEY DDLLALIADL ADILA KPER VSQIIKDELD EVKRKFSDKR RTELMVG UniProtKB: DNA gyrase subunit A |
-Macromolecule #2: DNA (44-MER)
| Macromolecule | Name: DNA (44-MER) / type: dna / ID: 2 / Number of copies: 1 / Classification: DNA |
|---|---|
| Source (natural) | Organism: Cloning vector pBR322 (others) |
| Molecular weight | Theoretical: 13.641771 KDa |
| Sequence | String: (DG)(DA)(DG)(DA)(DA)(DG)(DA)(DA)(DT)(DC) (DA)(DT)(DA)(DA)(DT)(DG)(DG)(DG)(DG)(DA) (DA)(DG)(DG)(DC)(DC)(DA)(DT)(DC)(DC) (DA)(DG)(DC)(DC)(DT)(DC)(DG)(DC)(DG)(DT) (DC) (DG)(DC)(DG)(DA) |
-Macromolecule #3: DNA (44-MER)
| Macromolecule | Name: DNA (44-MER) / type: dna / ID: 3 / Number of copies: 1 / Classification: DNA |
|---|---|
| Source (natural) | Organism: Cloning vector pBR322 (others) |
| Molecular weight | Theoretical: 13.4586 KDa |
| Sequence | String: (DT)(DC)(DG)(DC)(DG)(DA)(DC)(DG)(DC)(DG) (DA)(DG)(DG)(DC)(DT)(DG)(DG)(DA)(DT)(DG) (DG)(DC)(DC)(DT)(DT)(DC)(DC)(DC)(DC) (DA)(DT)(DT)(DA)(DT)(DG)(DA)(DT)(DT)(DC) (DT) (DT)(DC)(DT)(DC) |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Buffer | pH: 7.5 Component:
| ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Grid | Model: C-flat-1.2/1.3 4C / Material: COPPER / Mesh: 400 / Support film - Material: CARBON / Pretreatment - Type: GLOW DISCHARGE | ||||||||||||
| Vitrification | Cryogen name: ETHANE / Chamber humidity: 100 % / Chamber temperature: 277 K / Instrument: FEI VITROBOT MARK IV |
- Electron microscopy
Electron microscopy
| Microscope | JEOL 3200FS |
|---|---|
| Temperature | Min: 100.0 K / Max: 100.0 K |
| Specialist optics | Energy filter - Name: In-column Omega Filter / Energy filter - Slit width: 20 eV |
| Image recording | Film or detector model: GATAN K2 SUMMIT (4k x 4k) / Detector mode: COUNTING / Digitization - Dimensions - Width: 3710 pixel / Digitization - Dimensions - Height: 3838 pixel / Digitization - Frames/image: 1-40 / Number real images: 718 / Average exposure time: 12.0 sec. / Average electron dose: 62.0 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | C2 aperture diameter: 50.0 µm / Calibrated magnification: 40323 / Illumination mode: OTHER / Imaging mode: BRIGHT FIELD / Cs: 2.0 mm / Nominal defocus max: 3.0 µm / Nominal defocus min: 1.0 µm / Nominal magnification: 30000 |
| Sample stage | Specimen holder model: GATAN 626 SINGLE TILT LIQUID NITROGEN CRYO TRANSFER HOLDER Cooling holder cryogen: NITROGEN |
+ Image processing
Image processing
-Atomic model buiding 1
| Initial model | PDB ID: Chain - Chain ID: A / Chain - Residue range: 2-486 / Chain - Source name: PDB / Chain - Initial model type: experimental model |
|---|---|
| Refinement | Space: REAL / Protocol: FLEXIBLE FIT / Target criteria: correlation coefficient |
| Output model |  PDB-6n1p: |
 Movie
Movie Controller
Controller














 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)