[English] 日本語
 Yorodumi
Yorodumi- EMDB-9122: Flagellar motor averaged map from Borrelia burdorferi reveals the... -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: EMDB / ID: EMD-9122 | |||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Title | Flagellar motor averaged map from Borrelia burdorferi reveals the cytoplasmic ATPase complex | |||||||||||||||||||||
 Map data Map data | A recombined map of Borrelia burgdorferi flagellar motor (WT). The top part (collar and stators) and bottom part (C-ring and ATPase complex) have been processed separately. | |||||||||||||||||||||
 Sample Sample |
| |||||||||||||||||||||
| Biological species |  Borrelia burgdorferi (Lyme disease spirochete) Borrelia burgdorferi (Lyme disease spirochete) | |||||||||||||||||||||
| Method | subtomogram averaging / cryo EM / Resolution: 29.0 Å | |||||||||||||||||||||
 Authors Authors | Qin Z / Liu J | |||||||||||||||||||||
| Funding support |  United States, 6 items United States, 6 items
| |||||||||||||||||||||
 Citation Citation |  Journal: PLoS Biol / Year: 2018 Journal: PLoS Biol / Year: 2018Title: Cryo-electron tomography of periplasmic flagella in Borrelia burgdorferi reveals a distinct cytoplasmic ATPase complex. Authors: Zhuan Qin / Jiagang Tu / Tao Lin / Steven J Norris / Chunhao Li / Md A Motaleb / Jun Liu /  Abstract: Periplasmic flagella are essential for the distinct morphology and motility of spirochetes. A flagella-specific type III secretion system (fT3SS) composed of a membrane-bound export apparatus and a ...Periplasmic flagella are essential for the distinct morphology and motility of spirochetes. A flagella-specific type III secretion system (fT3SS) composed of a membrane-bound export apparatus and a cytosolic ATPase complex is responsible for the assembly of the periplasmic flagella. Here, we deployed cryo-electron tomography (cryo-ET) to visualize the fT3SS machine in the Lyme disease spirochete Borrelia burgdorferi. We show, for the first time, that the cytosolic ATPase complex is attached to the flagellar C-ring through multiple spokes to form the "spoke and hub" structure in B. burgdorferi. This structure not only strengthens structural rigidity of the round-shaped C-ring but also appears to rotate with the C-ring. Our studies provide structural insights into the unique mechanisms underlying assembly and rotation of the periplasmic flagella and may provide the basis for the development of novel therapeutic strategies against several pathogenic spirochetes. | |||||||||||||||||||||
| History |
|
- Structure visualization
Structure visualization
| Movie |
 Movie viewer Movie viewer |
|---|---|
| Structure viewer | EM map:  SurfView SurfView Molmil Molmil Jmol/JSmol Jmol/JSmol |
| Supplemental images |
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_9122.map.gz emd_9122.map.gz | 5.7 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-9122-v30.xml emd-9122-v30.xml emd-9122.xml emd-9122.xml | 15.7 KB 15.7 KB | Display Display |  EMDB header EMDB header |
| Images |  emd_9122.png emd_9122.png | 166.7 KB | ||
| Masks |  emd_9122_msk_1.map emd_9122_msk_1.map | 30.5 MB |  Mask map Mask map | |
| Others |  emd_9122_additional_1.map.gz emd_9122_additional_1.map.gz emd_9122_additional_2.map.gz emd_9122_additional_2.map.gz | 27.8 MB 27.8 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-9122 http://ftp.pdbj.org/pub/emdb/structures/EMD-9122 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-9122 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-9122 | HTTPS FTP |
-Validation report
| Summary document |  emd_9122_validation.pdf.gz emd_9122_validation.pdf.gz | 79.1 KB | Display |  EMDB validaton report EMDB validaton report |
|---|---|---|---|---|
| Full document |  emd_9122_full_validation.pdf.gz emd_9122_full_validation.pdf.gz | 78.2 KB | Display | |
| Data in XML |  emd_9122_validation.xml.gz emd_9122_validation.xml.gz | 493 B | Display | |
| Arichive directory |  https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-9122 https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-9122 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-9122 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-9122 | HTTPS FTP |
-Related structure data
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|
- Map
Map
| File |  Download / File: emd_9122.map.gz / Format: CCP4 / Size: 30.5 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_9122.map.gz / Format: CCP4 / Size: 30.5 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | A recombined map of Borrelia burgdorferi flagellar motor (WT). The top part (collar and stators) and bottom part (C-ring and ATPase complex) have been processed separately. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 5.208 Å | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
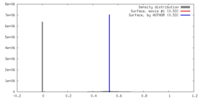
| Density |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
CCP4 map header:
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
-Supplemental data
-Mask #1
| File |  emd_9122_msk_1.map emd_9122_msk_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
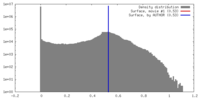
| Density Histograms |
-Additional map: An averaged map focus on linkers and C-ring,...
| File | emd_9122_additional_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | An averaged map focus on linkers and C-ring, showing 23 linkers and 46-fold symmetry of the C-ring. The ATPase is 6-fold symmetry. | ||||||||||||
| Projections & Slices |
| ||||||||||||
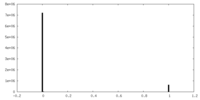
| Density Histograms |
-Additional map: The original averaged map with a focus on...
| File | emd_9122_additional_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | The original averaged map with a focus on the ATPase complex region. The map was not masked or filtered. The feature of the top part (collar and stators) was averaged out. | ||||||||||||
| Projections & Slices |
| ||||||||||||
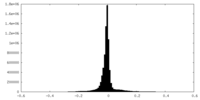
| Density Histograms |
- Sample components
Sample components
-Entire : Borrelia burgdorferi flagellar motor averaged recombined averaged...
| Entire | Name: Borrelia burgdorferi flagellar motor averaged recombined averaged map. It shows 16-fold symmetry at collar and stators region, 23 spoke linkers, and 6-fold symmetry of the ATPase. The top part ...Name: Borrelia burgdorferi flagellar motor averaged recombined averaged map. It shows 16-fold symmetry at collar and stators region, 23 spoke linkers, and 6-fold symmetry of the ATPase. The top part (collar and stators) and bottom part (C-ring and ATPase complex) were processed separatedly. |
|---|---|
| Components |
|
-Supramolecule #1: Borrelia burgdorferi flagellar motor averaged recombined averaged...
| Supramolecule | Name: Borrelia burgdorferi flagellar motor averaged recombined averaged map. It shows 16-fold symmetry at collar and stators region, 23 spoke linkers, and 6-fold symmetry of the ATPase. The top part ...Name: Borrelia burgdorferi flagellar motor averaged recombined averaged map. It shows 16-fold symmetry at collar and stators region, 23 spoke linkers, and 6-fold symmetry of the ATPase. The top part (collar and stators) and bottom part (C-ring and ATPase complex) were processed separatedly. type: organelle_or_cellular_component / ID: 1 / Parent: 0 Details: Wild-type. Top part and bottom part have different symmetry, and were processed separately from the same group of sub-tomograms. |
|---|---|
| Source (natural) | Organism:  Borrelia burgdorferi (Lyme disease spirochete) / Strain: B31 / Organelle: Flagellar motor / Location in cell: cell tip Borrelia burgdorferi (Lyme disease spirochete) / Strain: B31 / Organelle: Flagellar motor / Location in cell: cell tip |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | subtomogram averaging |
| Aggregation state | cell |
- Sample preparation
Sample preparation
| Buffer | pH: 7 Details: BSK-II liquid medium supplemented with 6% rabbit serum |
|---|---|
| Grid | Material: COPPER / Support film - Material: CARBON / Support film - topology: HOLEY / Pretreatment - Type: GLOW DISCHARGE |
| Vitrification | Cryogen name: ETHANE / Instrument: HOMEMADE PLUNGER |
- Electron microscopy
Electron microscopy
| Microscope | FEI POLARA 300 |
|---|---|
| Image recording | Film or detector model: GATAN K2 SUMMIT (4k x 4k) / Average electron dose: 100.0 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD |
| Experimental equipment |  Model: Tecnai Polara / Image courtesy: FEI Company |
- Image processing
Image processing
| Final reconstruction | Applied symmetry - Point group: C1 (asymmetric) / Resolution.type: BY AUTHOR / Resolution: 29.0 Å / Resolution method: FSC 0.143 CUT-OFF Details: It is combined from top map and bottom map. Top had 16-fold symmetry applied. The bottom map was asymmetric. Number subtomograms used: 4825 |
|---|---|
| Extraction | Number tomograms: 780 / Number images used: 6567 / Method: Subvolume averaging and Classification / Details: i3 |
| Final angle assignment | Type: OTHER |
 Movie
Movie Controller
Controller









 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)