[English] 日本語
 Yorodumi
Yorodumi- EMDB-8886: Cryo-EM structure of F-actin complexed with the beta-III-spectrin... -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: EMDB / ID: EMD-8886 | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
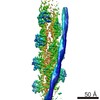
| Title | Cryo-EM structure of F-actin complexed with the beta-III-spectrin actin-binding domain | ||||||||||||
 Map data Map data | Cryo-EM structure of F-actin complexed with the beta-III-spectrin actin-binding domain | ||||||||||||
 Sample Sample |
| ||||||||||||
 Keywords Keywords | actin binding protein / filament / STRUCTURAL PROTEIN | ||||||||||||
| Function / homology |  Function and homology information Function and homology informationstructural constituent of synapse / postsynaptic spectrin-associated cytoskeleton / structural constituent of postsynapse / cerebellar Purkinje cell layer morphogenesis / spectrin / regulation of postsynaptic specialization assembly / positive regulation of norepinephrine uptake / paranodal junction / Regulation of CDH1 Function / Formation of the polybromo-BAF (pBAF) complex ...structural constituent of synapse / postsynaptic spectrin-associated cytoskeleton / structural constituent of postsynapse / cerebellar Purkinje cell layer morphogenesis / spectrin / regulation of postsynaptic specialization assembly / positive regulation of norepinephrine uptake / paranodal junction / Regulation of CDH1 Function / Formation of the polybromo-BAF (pBAF) complex / Formation of the non-canonical BAF (ncBAF) complex / Formation of the canonical BAF (cBAF) complex / Formation of the embryonic stem cell BAF (esBAF) complex / Formation of neuronal progenitor and neuronal BAF (npBAF and nBAF) / bBAF complex / cellular response to cytochalasin B / npBAF complex / nBAF complex / brahma complex / regulation of transepithelial transport / morphogenesis of a polarized epithelium / Formation of annular gap junctions / Formation of the dystrophin-glycoprotein complex (DGC) / structural constituent of postsynaptic actin cytoskeleton / GBAF complex / Gap junction degradation / Folding of actin by CCT/TriC / regulation of G0 to G1 transition / Cell-extracellular matrix interactions / protein localization to adherens junction / actin filament capping / dense body / Tat protein binding / postsynaptic actin cytoskeleton / Prefoldin mediated transfer of substrate to CCT/TriC / RSC-type complex / regulation of double-strand break repair / regulation of nucleotide-excision repair / Adherens junctions interactions / RHOF GTPase cycle / adherens junction assembly / apical protein localization / Sensory processing of sound by inner hair cells of the cochlea / Sensory processing of sound by outer hair cells of the cochlea / Interaction between L1 and Ankyrins / tight junction / regulation of mitotic metaphase/anaphase transition / SWI/SNF complex / positive regulation of T cell differentiation / apical junction complex / cortical actin cytoskeleton / positive regulation of double-strand break repair / maintenance of blood-brain barrier / regulation of norepinephrine uptake / transporter regulator activity / nitric-oxide synthase binding / cortical cytoskeleton / adult behavior / positive regulation of stem cell population maintenance / NuA4 histone acetyltransferase complex / establishment or maintenance of cell polarity / parallel fiber to Purkinje cell synapse / Recycling pathway of L1 / Regulation of MITF-M-dependent genes involved in pigmentation / brush border / regulation of G1/S transition of mitotic cell cycle / EPH-ephrin mediated repulsion of cells / negative regulation of cell differentiation / kinesin binding / RHO GTPases Activate WASPs and WAVEs / regulation of synaptic vesicle endocytosis / positive regulation of myoblast differentiation / RHO GTPases activate IQGAPs / regulation of protein localization to plasma membrane / positive regulation of double-strand break repair via homologous recombination / COPI-mediated anterograde transport / vesicle-mediated transport / synapse assembly / EPHB-mediated forward signaling / cytoskeleton organization / NCAM signaling for neurite out-growth / MHC class II antigen presentation / substantia nigra development / axonogenesis / calyx of Held / cell projection / nitric-oxide synthase regulator activity / FCGR3A-mediated phagocytosis / adherens junction / Translocation of SLC2A4 (GLUT4) to the plasma membrane / actin filament / positive regulation of cell differentiation / cell motility / Regulation of endogenous retroelements by Piwi-interacting RNAs (piRNAs) / Signaling by high-kinase activity BRAF mutants / RHO GTPases Activate Formins / MAP2K and MAPK activation / phospholipid binding / Regulation of actin dynamics for phagocytic cup formation / kinetochore Similarity search - Function | ||||||||||||
| Biological species |  Homo sapiens (human) Homo sapiens (human) | ||||||||||||
| Method | helical reconstruction / cryo EM / negative staining / Resolution: 7.0 Å | ||||||||||||
 Authors Authors | Wang F / Orlova A | ||||||||||||
| Funding support |  United States, 3 items United States, 3 items
| ||||||||||||
 Citation Citation |  Journal: Nat Commun / Year: 2017 Journal: Nat Commun / Year: 2017Title: Structural basis for high-affinity actin binding revealed by a β-III-spectrin SCA5 missense mutation. Authors: Adam W Avery / Michael E Fealey / Fengbin Wang / Albina Orlova / Andrew R Thompson / David D Thomas / Thomas S Hays / Edward H Egelman /  Abstract: Spinocerebellar ataxia type 5 (SCA5) is a neurodegenerative disease caused by mutations in the cytoskeletal protein β-III-spectrin. Previously, a SCA5 mutation resulting in a leucine-to-proline ...Spinocerebellar ataxia type 5 (SCA5) is a neurodegenerative disease caused by mutations in the cytoskeletal protein β-III-spectrin. Previously, a SCA5 mutation resulting in a leucine-to-proline substitution (L253P) in the actin-binding domain (ABD) was shown to cause a 1000-fold increase in actin-binding affinity. However, the structural basis for this increase is unknown. Here, we report a 6.9 Å cryo-EM structure of F-actin complexed with the L253P ABD. This structure, along with co-sedimentation and pulsed-EPR measurements, demonstrates that high-affinity binding caused by the CH2-localized mutation is due to opening of the two CH domains. This enables CH1 to bind actin aided by an unstructured N-terminal region that becomes α-helical upon binding. This helix is required for association with actin as truncation eliminates binding. Collectively, these results shed light on the mechanism by which β-III-spectrin, and likely similar actin-binding proteins, interact with actin, and how this mechanism can be perturbed to cause disease. | ||||||||||||
| History |
|
- Structure visualization
Structure visualization
| Movie |
 Movie viewer Movie viewer |
|---|---|
| Structure viewer | EM map:  SurfView SurfView Molmil Molmil Jmol/JSmol Jmol/JSmol |
| Supplemental images |
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_8886.map.gz emd_8886.map.gz | 24.6 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-8886-v30.xml emd-8886-v30.xml emd-8886.xml emd-8886.xml | 12.7 KB 12.7 KB | Display Display |  EMDB header EMDB header |
| Images |  emd_8886.png emd_8886.png | 180.3 KB | ||
| Filedesc metadata |  emd-8886.cif.gz emd-8886.cif.gz | 5.8 KB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-8886 http://ftp.pdbj.org/pub/emdb/structures/EMD-8886 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-8886 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-8886 | HTTPS FTP |
-Related structure data
| Related structure data |  6anuMC M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_8886.map.gz / Format: CCP4 / Size: 30.5 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_8886.map.gz / Format: CCP4 / Size: 30.5 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Cryo-EM structure of F-actin complexed with the beta-III-spectrin actin-binding domain | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 1.05 Å | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Density |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
CCP4 map header:
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
-Supplemental data
- Sample components
Sample components
-Entire : F-actin complexed with the spectrin actin-binding domain
| Entire | Name: F-actin complexed with the spectrin actin-binding domain |
|---|---|
| Components |
|
-Supramolecule #1: F-actin complexed with the spectrin actin-binding domain
| Supramolecule | Name: F-actin complexed with the spectrin actin-binding domain type: complex / ID: 1 / Parent: 0 / Macromolecule list: all |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
-Macromolecule #1: Actin, cytoplasmic 1
| Macromolecule | Name: Actin, cytoplasmic 1 / type: protein_or_peptide / ID: 1 / Number of copies: 6 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 41.78266 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: MDDDIAALVV DNGSGMCKAG FAGDDAPRAV FPSIVGRPRH QGVMVGMGQK DSYVGDEAQS KRGILTLKYP IEHGIVTNWD DMEKIWHHT FYNELRVAPE EHPVLLTEAP LNPKANREKM TQIMFETFNT PAMYVAIQAV LSLYASGRTT GIVMDSGDGV T HTVPIYEG ...String: MDDDIAALVV DNGSGMCKAG FAGDDAPRAV FPSIVGRPRH QGVMVGMGQK DSYVGDEAQS KRGILTLKYP IEHGIVTNWD DMEKIWHHT FYNELRVAPE EHPVLLTEAP LNPKANREKM TQIMFETFNT PAMYVAIQAV LSLYASGRTT GIVMDSGDGV T HTVPIYEG YALPHAILRL DLAGRDLTDY LMKILTERGY SFTTTAEREI VRDIKEKLCY VALDFEQEMA TAASSSSLEK SY ELPDGQV ITIGNERFRC PEALFQPSFL GMESCGIHET TFNSIMKCDV DIRKDLYANT VLSGGTTMYP GIADRMQKEI TAL APSTMK IKIIAPPERK YSVWIGGSIL ASLSTFQQMW ISKQEYDESG PSIVHRKCF UniProtKB: Actin, cytoplasmic 1 |
-Macromolecule #2: Spectrin beta chain, non-erythrocytic 2
| Macromolecule | Name: Spectrin beta chain, non-erythrocytic 2 / type: protein_or_peptide / ID: 2 / Number of copies: 6 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 32.857141 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: MSSTLSPTDF DSLEIQGQYS DINNRWDLPD SDWDNDSSSA RLFERSRIKA LADEREAVQK KTFTKWVNSH LARVTCRVGD LYSDLRDGR NLLRLLEVLS GEILPKPTKG RMRIHCLENV DKALQFLKEQ KVHLENMGSH DIVDGNHRLT LGLVWTIILR F QIQDISVE ...String: MSSTLSPTDF DSLEIQGQYS DINNRWDLPD SDWDNDSSSA RLFERSRIKA LADEREAVQK KTFTKWVNSH LARVTCRVGD LYSDLRDGR NLLRLLEVLS GEILPKPTKG RMRIHCLENV DKALQFLKEQ KVHLENMGSH DIVDGNHRLT LGLVWTIILR F QIQDISVE TEDNKEKKSA KDALLLWCQM KTAGYPNVNV HNFTTSWRDG LAFNAIVHKH RPDLLDFESL KKCNAHYNLQ NA FNLAEKE LGLTKPLDPE DVNVDQPDEK SIITYVATYY HYFSKMK UniProtKB: Spectrin beta chain, non-erythrocytic 2 |
-Experimental details
-Structure determination
| Method | negative staining, cryo EM |
|---|---|
 Processing Processing | helical reconstruction |
| Aggregation state | filament |
- Sample preparation
Sample preparation
| Buffer | pH: 7.4 |
|---|---|
| Staining | Type: NEGATIVE / Material: negative stain |
| Vitrification | Cryogen name: ETHANE |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Image recording | Film or detector model: FEI FALCON II (4k x 4k) / Detector mode: INTEGRATING / Average exposure time: 3.0 sec. / Average electron dose: 20.0 e/Å2 Details: Images were stored containing seven parts, where each part represented a set of frames corresponding to a dose of ~20 electrons per Angstrom^2. The full dose image stack was used for the ...Details: Images were stored containing seven parts, where each part represented a set of frames corresponding to a dose of ~20 electrons per Angstrom^2. The full dose image stack was used for the estimation of the CTF as well as for boxing filaments. Only the first two parts were used for the reconstruction (~5 electrons per Angstrom^2). |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
- Image processing
Image processing
| Final reconstruction | Applied symmetry - Helical parameters - Δz: 27.25 Å Applied symmetry - Helical parameters - Δ&Phi: -166.87 ° Applied symmetry - Helical parameters - Axial symmetry: C1 (asymmetric) Algorithm: BACK PROJECTION / Resolution.type: BY AUTHOR / Resolution: 7.0 Å / Resolution method: OTHER / Software - Name: SPIDER / Details: model-map FSC 0.38 cut-off / Number images used: 12443 |
|---|---|
| Startup model | Type of model: OTHER / Details: low resolution pure actin filament map |
| Final angle assignment | Type: NOT APPLICABLE / Software - Name: SPIDER |
-Atomic model buiding 1
| Refinement | Space: REAL |
|---|---|
| Output model |  PDB-6anu: |
 Movie
Movie Controller
Controller




































 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)





















