[English] 日本語
 Yorodumi
Yorodumi- EMDB-8742: Thermoplasma acidophilum 20S Proteasome using 200keV with image shift -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: EMDB / ID: EMD-8742 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | Thermoplasma acidophilum 20S Proteasome using 200keV with image shift | |||||||||
 Map data Map data | Final sharpened map of T. acidophilum 20S proteasome collected using image shift navigation | |||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | Proteasome / hydrolase | |||||||||
| Function / homology |  Function and homology information Function and homology informationproteasome endopeptidase complex / proteasome core complex, beta-subunit complex / threonine-type endopeptidase activity / proteasome core complex, alpha-subunit complex / proteasomal protein catabolic process / endopeptidase activity / ubiquitin-dependent protein catabolic process / cytoplasm Similarity search - Function | |||||||||
| Biological species |   Thermoplasma acidophilum (acidophilic) Thermoplasma acidophilum (acidophilic) | |||||||||
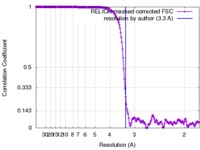
| Method | single particle reconstruction / cryo EM / Resolution: 3.3 Å | |||||||||
 Authors Authors | Herzik Jr MA / Wu M / Lander GC | |||||||||
| Funding support |  United States, 1 items United States, 1 items
| |||||||||
 Citation Citation |  Journal: Nat Methods / Year: 2017 Journal: Nat Methods / Year: 2017Title: Achieving better-than-3-Å resolution by single-particle cryo-EM at 200 keV. Authors: Mark A Herzik / Mengyu Wu / Gabriel C Lander /  Abstract: Nearly all single-particle cryo-EM structures resolved to better than 4-Å resolution have been determined using 300-keV transmission electron microscopes (TEMs). We demonstrate that it is possible ...Nearly all single-particle cryo-EM structures resolved to better than 4-Å resolution have been determined using 300-keV transmission electron microscopes (TEMs). We demonstrate that it is possible to obtain reconstructions of macromolecular complexes of different sizes to better than 3-Å resolution using a 200-keV TEM. These structures are of sufficient quality to unambiguously assign amino acid rotameric conformations and identify ordered water molecules. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Movie |
 Movie viewer Movie viewer |
|---|---|
| Structure viewer | EM map:  SurfView SurfView Molmil Molmil Jmol/JSmol Jmol/JSmol |
| Supplemental images |
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_8742.map.gz emd_8742.map.gz | 59.5 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-8742-v30.xml emd-8742-v30.xml emd-8742.xml emd-8742.xml | 22.5 KB 22.5 KB | Display Display |  EMDB header EMDB header |
| FSC (resolution estimation) |  emd_8742_fsc.xml emd_8742_fsc.xml | 18 KB | Display |  FSC data file FSC data file |
| Images |  emd_8742.png emd_8742.png | 38.3 KB | ||
| Filedesc metadata |  emd-8742.cif.gz emd-8742.cif.gz | 6.6 KB | ||
| Others |  emd_8742_additional.map.gz emd_8742_additional.map.gz emd_8742_half_map_1.map.gz emd_8742_half_map_1.map.gz emd_8742_half_map_2.map.gz emd_8742_half_map_2.map.gz | 59.1 MB 409.7 MB 409.7 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-8742 http://ftp.pdbj.org/pub/emdb/structures/EMD-8742 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-8742 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-8742 | HTTPS FTP |
-Related structure data
| Related structure data |  5vy4MC  8741C  8743C  5vy3C  5vy5C C: citing same article ( M: atomic model generated by this map |
|---|---|
| Similar structure data | |
| EM raw data |  EMPIAR-10186 (Title: T. acidophilum 20S proteasome core movies obtained using Talos Arctica operating at 200 kV equipped with a K2 – image shift used for exposure target navigation EMPIAR-10186 (Title: T. acidophilum 20S proteasome core movies obtained using Talos Arctica operating at 200 kV equipped with a K2 – image shift used for exposure target navigationData size: 945.5 Data #1: Raw, unaligned movie stacks of T. acidophilum 20S proteasome core acquired on a Talos Arctica using a K2 direct electron detector - image shift used for exposure navigation [micrographs - multiframe]) |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_8742.map.gz / Format: CCP4 / Size: 64 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_8742.map.gz / Format: CCP4 / Size: 64 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Final sharpened map of T. acidophilum 20S proteasome collected using image shift navigation | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 0.91 Å | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
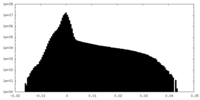
| Density |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
CCP4 map header:
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
-Supplemental data
-Additional map: Unsharpened map of T. acidophilum 20S proteasome collected...
| File | emd_8742_additional.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Unsharpened map of T. acidophilum 20S proteasome collected using image shift navigation | ||||||||||||
| Projections & Slices |
| ||||||||||||
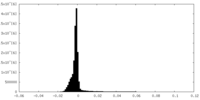
| Density Histograms |
-Half map: Thermoplasma acidophilum 20S Proteasome, even half map
| File | emd_8742_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Thermoplasma acidophilum 20S Proteasome, even half map | ||||||||||||
| Projections & Slices |
| ||||||||||||
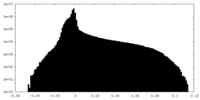
| Density Histograms |
-Half map: Thermoplasma acidophilum 20S Proteasome, odd half map
| File | emd_8742_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Thermoplasma acidophilum 20S Proteasome, odd half map | ||||||||||||
| Projections & Slices |
| ||||||||||||
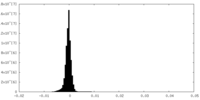
| Density Histograms |
- Sample components
Sample components
-Entire : Thermoplasma acidophilum 20S proteasome
| Entire | Name: Thermoplasma acidophilum 20S proteasome |
|---|---|
| Components |
|
-Supramolecule #1: Thermoplasma acidophilum 20S proteasome
| Supramolecule | Name: Thermoplasma acidophilum 20S proteasome / type: complex / ID: 1 / Parent: 0 / Macromolecule list: all Details: Thermoplasma acidophilum 20S proteasome purified from Escherichia coli |
|---|---|
| Source (natural) | Organism:   Thermoplasma acidophilum (acidophilic) Thermoplasma acidophilum (acidophilic) |
| Molecular weight | Theoretical: 700 KDa |
-Macromolecule #1: Proteasome subunit alpha
| Macromolecule | Name: Proteasome subunit alpha / type: protein_or_peptide / ID: 1 / Number of copies: 14 / Enantiomer: LEVO / EC number: proteasome endopeptidase complex |
|---|---|
| Source (natural) | Organism:   Thermoplasma acidophilum (acidophilic) Thermoplasma acidophilum (acidophilic) |
| Molecular weight | Theoretical: 24.776281 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: RAITVFSPDG RLFQVEYARE AVKKGSTALG MKFANGVLLI SDKKVRSRLI EQNSIEKIQL IDDYVAAVTS GLVADARVLV DFARISAQQ EKVTYGSLVN IENLVKRVAD QMQQYTQYGG VRPYGVSLIF AGIDQIGPRL FDCDPAGTIN EYKATAIGSG K DAVVSFLE ...String: RAITVFSPDG RLFQVEYARE AVKKGSTALG MKFANGVLLI SDKKVRSRLI EQNSIEKIQL IDDYVAAVTS GLVADARVLV DFARISAQQ EKVTYGSLVN IENLVKRVAD QMQQYTQYGG VRPYGVSLIF AGIDQIGPRL FDCDPAGTIN EYKATAIGSG K DAVVSFLE REYKENLPEK EAVTLGIKAL KSSLEEGEEL KAPEIASITV GNKYRIYDQE EVKKFL UniProtKB: Proteasome subunit alpha |
-Macromolecule #2: Proteasome subunit beta
| Macromolecule | Name: Proteasome subunit beta / type: protein_or_peptide / ID: 2 / Number of copies: 14 / Enantiomer: LEVO / EC number: proteasome endopeptidase complex |
|---|---|
| Source (natural) | Organism:   Thermoplasma acidophilum (acidophilic) Thermoplasma acidophilum (acidophilic) |
| Molecular weight | Theoretical: 22.294848 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: TTTVGITLKD AVIMATERRV TMENFIMHKN GKKLFQIDTY TGMTIAGLVG DAQVLVRYMK AELELYRLQR RVNMPIEAVA TLLSNMLNQ VKYMPYMVQL LVGGIDTAPH VFSIDAAGGS VEDIYASTGS GSPFVYGVLE SQYSEKMTVD EGVDLVIRAI S AAKQRDSA ...String: TTTVGITLKD AVIMATERRV TMENFIMHKN GKKLFQIDTY TGMTIAGLVG DAQVLVRYMK AELELYRLQR RVNMPIEAVA TLLSNMLNQ VKYMPYMVQL LVGGIDTAPH VFSIDAAGGS VEDIYASTGS GSPFVYGVLE SQYSEKMTVD EGVDLVIRAI S AAKQRDSA SGGMIDVAVI TRKDGYVQLP TDQIESRIRK LGLIL UniProtKB: Proteasome subunit beta |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Concentration | 0.5 mg/mL | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Buffer | pH: 7.4 Component:
| |||||||||
| Grid | Model: Quantifoil, UltrAuFoil, R1.2/1.3 / Material: GOLD / Mesh: 300 / Pretreatment - Type: PLASMA CLEANING / Pretreatment - Time: 7 sec. / Pretreatment - Atmosphere: OTHER / Pretreatment - Pressure: 0.009000000000000001 kPa / Details: 15 Watts | |||||||||
| Vitrification | Cryogen name: ETHANE / Chamber humidity: 100 % / Chamber temperature: 277.15 K / Instrument: HOMEMADE PLUNGER Details: 3 uL of sample/grid was manually blotted for 4 seconds prior to immediate plunge-freezing in liquid nitrogen-cooled ethane.. |
- Electron microscopy
Electron microscopy
| Microscope | FEI TALOS ARCTICA |
|---|---|
| Image recording | Film or detector model: GATAN K2 SUMMIT (4k x 4k) / Detector mode: SUPER-RESOLUTION / Digitization - Dimensions - Width: 7420 pixel / Digitization - Dimensions - Height: 7676 pixel / Digitization - Frames/image: 1-68 / Number grids imaged: 1 / Number real images: 394 / Average exposure time: 17.0 sec. / Average electron dose: 65.0 e/Å2 Details: Images were collected using image shift navigation to target exposure. |
| Electron beam | Acceleration voltage: 200 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | C2 aperture diameter: 70.0 µm / Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD / Cs: 2.7 mm / Nominal defocus max: 2.8000000000000003 µm / Nominal defocus min: 0.8 µm / Nominal magnification: 45000 |
| Sample stage | Specimen holder model: FEI TITAN KRIOS AUTOGRID HOLDER / Cooling holder cryogen: NITROGEN |
| Experimental equipment |  Model: Talos Arctica / Image courtesy: FEI Company |
+ Image processing
Image processing
-Atomic model buiding 1
| Details | Starting model was generated by stripping PDB entry 1YAR of all ligands and alternate conformations, then refining into the EM density using imposed symmetry while adjusting weighting/scoring according to estimated map resolution. The top 10 generated models (ranked based on quality metrics) were real-space refined using Phenix software. |
|---|---|
| Refinement | Space: REAL / Protocol: OTHER / Overall B value: 99 / Target criteria: Maximum Likelihood |
| Output model |  PDB-5vy4: |
 Movie
Movie Controller
Controller














 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)