[English] 日本語
 Yorodumi
Yorodumi- EMDB-8617: 70S ribosome bound with cognate ternary complex base-paired to A ... -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: EMDB / ID: EMD-8617 | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Title | 70S ribosome bound with cognate ternary complex base-paired to A site codon, closed 30S (Structure III) | |||||||||||||||
 Map data Map data | 70S ribosome bound with cognate ternary complex base-paired to A site codon, closed 30S (Structure III): filtered map from Frealign (FFILT setting in Frealign = T), used for model building after B-factor sharpening. This volume not B-factor sharpened. | |||||||||||||||
 Sample Sample |
| |||||||||||||||
 Keywords Keywords | ribosome / ternary complex | |||||||||||||||
| Function / homology |  Function and homology information Function and homology informationguanyl-nucleotide exchange factor complex / protein-synthesizing GTPase / guanosine tetraphosphate binding / negative regulation of cytoplasmic translational initiation / stringent response / transcription antitermination factor activity, RNA binding / ornithine decarboxylase inhibitor activity / misfolded RNA binding / Group I intron splicing / translational elongation ...guanyl-nucleotide exchange factor complex / protein-synthesizing GTPase / guanosine tetraphosphate binding / negative regulation of cytoplasmic translational initiation / stringent response / transcription antitermination factor activity, RNA binding / ornithine decarboxylase inhibitor activity / misfolded RNA binding / Group I intron splicing / translational elongation / RNA folding / transcriptional attenuation / endoribonuclease inhibitor activity / translation elongation factor activity / positive regulation of ribosome biogenesis / RNA-binding transcription regulator activity / translational termination / negative regulation of cytoplasmic translation / four-way junction DNA binding / DnaA-L2 complex / translation repressor activity / negative regulation of translational initiation / regulation of mRNA stability / negative regulation of DNA-templated DNA replication initiation / mRNA regulatory element binding translation repressor activity / positive regulation of RNA splicing / assembly of large subunit precursor of preribosome / cytosolic ribosome assembly / response to reactive oxygen species / regulation of DNA-templated transcription elongation / ribosome assembly / transcription elongation factor complex / transcription antitermination / DNA endonuclease activity / regulation of cell growth / translational initiation / DNA-templated transcription termination / response to radiation / maintenance of translational fidelity / mRNA 5'-UTR binding / regulation of translation / large ribosomal subunit / ribosome biogenesis / transferase activity / ribosome binding / ribosomal small subunit biogenesis / ribosomal small subunit assembly / 5S rRNA binding / ribosomal large subunit assembly / small ribosomal subunit / small ribosomal subunit rRNA binding / large ribosomal subunit rRNA binding / cytosolic small ribosomal subunit / cytosolic large ribosomal subunit / cytoplasmic translation / tRNA binding / negative regulation of translation / rRNA binding / structural constituent of ribosome / ribosome / translation / hydrolase activity / response to antibiotic / negative regulation of DNA-templated transcription / GTPase activity / mRNA binding / GTP binding / magnesium ion binding / DNA binding / RNA binding / zinc ion binding / membrane / plasma membrane / cytosol / cytoplasm Similarity search - Function | |||||||||||||||
| Biological species |  | |||||||||||||||
| Method | single particle reconstruction / cryo EM / Resolution: 3.2 Å | |||||||||||||||
 Authors Authors | Loveland AB / Demo G | |||||||||||||||
| Funding support |  United States, 4 items United States, 4 items
| |||||||||||||||
 Citation Citation |  Journal: Nature / Year: 2017 Journal: Nature / Year: 2017Title: Ensemble cryo-EM elucidates the mechanism of translation fidelity. Authors: Anna B Loveland / Gabriel Demo / Nikolaus Grigorieff / Andrei A Korostelev /  Abstract: Gene translation depends on accurate decoding of mRNA, the structural mechanism of which remains poorly understood. Ribosomes decode mRNA codons by selecting cognate aminoacyl-tRNAs delivered by ...Gene translation depends on accurate decoding of mRNA, the structural mechanism of which remains poorly understood. Ribosomes decode mRNA codons by selecting cognate aminoacyl-tRNAs delivered by elongation factor Tu (EF-Tu). Here we present high-resolution structural ensembles of ribosomes with cognate or near-cognate aminoacyl-tRNAs delivered by EF-Tu. Both cognate and near-cognate tRNA anticodons explore the aminoacyl-tRNA-binding site (A site) of an open 30S subunit, while inactive EF-Tu is separated from the 50S subunit. A transient conformation of decoding-centre nucleotide G530 stabilizes the cognate codon-anticodon helix, initiating step-wise 'latching' of the decoding centre. The resulting closure of the 30S subunit docks EF-Tu at the sarcin-ricin loop of the 50S subunit, activating EF-Tu for GTP hydrolysis and enabling accommodation of the aminoacyl-tRNA. By contrast, near-cognate complexes fail to induce the G530 latch, thus favouring open 30S pre-accommodation intermediates with inactive EF-Tu. This work reveals long-sought structural differences between the pre-accommodation of cognate and near-cognate tRNAs that elucidate the mechanism of accurate decoding. | |||||||||||||||
| History |
|
- Structure visualization
Structure visualization
| Movie |
 Movie viewer Movie viewer |
|---|---|
| Structure viewer | EM map:  SurfView SurfView Molmil Molmil Jmol/JSmol Jmol/JSmol |
| Supplemental images |
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_8617.map.gz emd_8617.map.gz | 99.1 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-8617-v30.xml emd-8617-v30.xml emd-8617.xml emd-8617.xml | 85.8 KB 85.8 KB | Display Display |  EMDB header EMDB header |
| Images |  emd_8617.png emd_8617.png | 155.8 KB | ||
| Filedesc metadata |  emd-8617.cif.gz emd-8617.cif.gz | 15.3 KB | ||
| Others |  emd_8617_half_map_1.map.gz emd_8617_half_map_1.map.gz emd_8617_half_map_2.map.gz emd_8617_half_map_2.map.gz | 101.4 MB 101.4 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-8617 http://ftp.pdbj.org/pub/emdb/structures/EMD-8617 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-8617 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-8617 | HTTPS FTP |
-Related structure data
| Related structure data |  5uymMC  8615C  8616C  8618C  8619C  8620C  5uykC  5uylC  5uynC  5uypC  5uyqC C: citing same article ( M: atomic model generated by this map |
|---|---|
| Similar structure data |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_8617.map.gz / Format: CCP4 / Size: 421.9 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_8617.map.gz / Format: CCP4 / Size: 421.9 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | 70S ribosome bound with cognate ternary complex base-paired to A site codon, closed 30S (Structure III): filtered map from Frealign (FFILT setting in Frealign = T), used for model building after B-factor sharpening. This volume not B-factor sharpened. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 0.82 Å | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Density |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
CCP4 map header:
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
-Supplemental data
-Half map: 70S ribosome bound with cognate ternary complex base-paired...
| File | emd_8617_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | 70S ribosome bound with cognate ternary complex base-paired to A site codon, closed 30S (Structure III): half map (1) suitable for resolution calculation. | ||||||||||||
| Projections & Slices |
| ||||||||||||
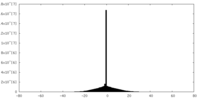
| Density Histograms |
-Half map: 70S ribosome bound with cognate ternary complex base-paired...
| File | emd_8617_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | 70S ribosome bound with cognate ternary complex base-paired to A site codon, closed 30S (Structure III): half map (2) suitable for resolution calculation. | ||||||||||||
| Projections & Slices |
| ||||||||||||
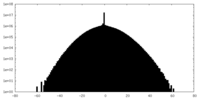
| Density Histograms |
- Sample components
Sample components
+Entire : 70S ribosome bound with cognate ternary complex base-paired to A ...
+Supramolecule #1: 70S ribosome bound with cognate ternary complex base-paired to A ...
+Macromolecule #1: 50S ribosomal protein L2
+Macromolecule #2: 50S ribosomal protein L3
+Macromolecule #3: 50S ribosomal protein L4
+Macromolecule #4: 50S ribosomal protein L5
+Macromolecule #5: 50S ribosomal protein L6
+Macromolecule #6: 50S ribosomal protein L9
+Macromolecule #7: 50S ribosomal protein L10
+Macromolecule #8: 50S ribosomal protein L11
+Macromolecule #9: 50S ribosomal protein L13
+Macromolecule #10: 50S ribosomal protein L14
+Macromolecule #11: 50S ribosomal protein L15
+Macromolecule #12: 50S ribosomal protein L16
+Macromolecule #13: 50S ribosomal protein L17
+Macromolecule #14: 50S ribosomal protein L18
+Macromolecule #15: 50S ribosomal protein L19
+Macromolecule #16: 50S ribosomal protein L20
+Macromolecule #17: 50S ribosomal protein L21
+Macromolecule #18: 50S ribosomal protein L22
+Macromolecule #19: 50S ribosomal protein L23
+Macromolecule #20: 50S ribosomal protein L24
+Macromolecule #21: 50S ribosomal protein L25
+Macromolecule #22: 50S ribosomal protein L27
+Macromolecule #23: 50S ribosomal protein L28
+Macromolecule #24: 50S ribosomal protein L29
+Macromolecule #25: 50S ribosomal protein L30
+Macromolecule #26: 50S ribosomal protein L31
+Macromolecule #27: 50S ribosomal protein L32
+Macromolecule #28: 50S ribosomal protein L33
+Macromolecule #29: 50S ribosomal protein L34
+Macromolecule #30: 50S ribosomal protein L35
+Macromolecule #31: 50S ribosomal protein L36
+Macromolecule #32: 30S ribosomal protein S2
+Macromolecule #33: 30S ribosomal protein S3
+Macromolecule #34: 30S ribosomal protein S4
+Macromolecule #35: 30S ribosomal protein S5
+Macromolecule #36: 30S ribosomal protein S6
+Macromolecule #37: 30S ribosomal protein S7
+Macromolecule #38: 30S ribosomal protein S8
+Macromolecule #39: 30S ribosomal protein S9
+Macromolecule #40: 30S ribosomal protein S10
+Macromolecule #41: 30S ribosomal protein S11
+Macromolecule #42: 30S ribosomal protein S12
+Macromolecule #43: 30S ribosomal protein S13
+Macromolecule #44: 30S ribosomal protein S14
+Macromolecule #45: 30S ribosomal protein S15
+Macromolecule #46: 30S ribosomal protein S16
+Macromolecule #47: 30S ribosomal protein S17
+Macromolecule #48: 30S ribosomal protein S18
+Macromolecule #49: 30S ribosomal protein S19
+Macromolecule #50: 30S ribosomal protein S20
+Macromolecule #51: 30S ribosomal protein S21
+Macromolecule #52: 50S ribosomal protein L1
+Macromolecule #59: Elongation factor Tu 2
+Macromolecule #53: 16S ribosomal RNA
+Macromolecule #54: 23S ribosomal RNA
+Macromolecule #55: 5S ribosomal RNA
+Macromolecule #56: tRNAfMet
+Macromolecule #57: mRNA
+Macromolecule #58: Phe-tRNAPhe
+Macromolecule #60: MAGNESIUM ION
+Macromolecule #61: ZINC ION
+Macromolecule #62: N-FORMYLMETHIONINE
+Macromolecule #63: PHENYLALANINE
+Macromolecule #64: PHOSPHOMETHYLPHOSPHONIC ACID GUANYLATE ESTER
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Buffer | pH: 7.5 Component:
| |||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Grid | Model: C-flat-1.2/1.3 / Material: COPPER / Mesh: 400 / Support film - Material: CARBON / Support film - topology: HOLEY ARRAY / Pretreatment - Type: GLOW DISCHARGE / Pretreatment - Time: 45 sec. / Pretreatment - Atmosphere: AIR | |||||||||||||||||||||
| Vitrification | Cryogen name: ETHANE / Chamber humidity: 90 % / Chamber temperature: 275 K / Instrument: GATAN CRYOPLUNGE 3 Details: 2 uL of complex was applied to each grid. After a 10-second incubation, the grids were blotted for 2 to 4 seconds.. | |||||||||||||||||||||
| Details | 250 nM 50S, 250 nM 30S, 1.25 micromolar mRNA, 500 nM fMet-tRNAfMet, 1 micromolar EF-T, 500 micromolar GDPCP, 1 micromolar Phe-tRNAPhe |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Image recording | Film or detector model: GATAN K2 SUMMIT (4k x 4k) / Detector mode: SUPER-RESOLUTION / Digitization - Dimensions - Width: 7676 pixel / Digitization - Dimensions - Height: 7420 pixel / Digitization - Frames/image: 1-50 / Number grids imaged: 2 / Number real images: 3928 / Average exposure time: 0.4 sec. / Average electron dose: 1.0 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Calibrated magnification: 60976 / Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD / Cs: 2.7 mm / Nominal defocus max: 5.0 µm / Nominal defocus min: 0.5 µm / Nominal magnification: 60976 |
| Sample stage | Specimen holder model: FEI TITAN KRIOS AUTOGRID HOLDER / Cooling holder cryogen: NITROGEN |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
+ Image processing
Image processing
-Atomic model buiding 1
| Refinement | Space: REAL / Protocol: FLEXIBLE FIT / Target criteria: correlation coefficient |
|---|---|
| Output model |  PDB-5uym: |
 Movie
Movie Controller
Controller






























 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)