[English] 日本語
 Yorodumi
Yorodumi- EMDB-8540: Structural basis of MCM2-7 replicative helicase loading by ORC-Cd... -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: EMDB / ID: EMD-8540 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
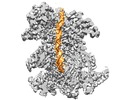
| Title | Structural basis of MCM2-7 replicative helicase loading by ORC-Cdc6 and Cdt1 | |||||||||
 Map data Map data | Tetrameric HIV-1 Strand Transfer Complex Intasome | |||||||||
 Sample Sample |
| |||||||||
| Function / homology |  Function and homology information Function and homology informationMCM complex loading / CDC6 association with the ORC:origin complex / Cul8-RING ubiquitin ligase complex / maintenance of rDNA / MCM core complex / Assembly of the pre-replicative complex / Switching of origins to a post-replicative state / MCM complex binding / nuclear DNA replication / premeiotic DNA replication ...MCM complex loading / CDC6 association with the ORC:origin complex / Cul8-RING ubiquitin ligase complex / maintenance of rDNA / MCM core complex / Assembly of the pre-replicative complex / Switching of origins to a post-replicative state / MCM complex binding / nuclear DNA replication / premeiotic DNA replication / Assembly of the ORC complex at the origin of replication / replication fork protection complex / pre-replicative complex assembly involved in nuclear cell cycle DNA replication / nuclear origin of replication recognition complex / Activation of the pre-replicative complex / mitotic DNA replication / CMG complex / nuclear pre-replicative complex / cyclin-dependent protein serine/threonine kinase inhibitor activity / DNA replication preinitiation complex / Activation of ATR in response to replication stress / MCM complex / nucleosome organization / mitotic DNA replication checkpoint signaling / double-strand break repair via break-induced replication / mitotic DNA replication initiation / single-stranded DNA helicase activity / silent mating-type cassette heterochromatin formation / regulation of DNA-templated DNA replication initiation / CDK-mediated phosphorylation and removal of Cdc6 / DNA strand elongation involved in DNA replication / Orc1 removal from chromatin / nuclear replication fork / regulation of DNA replication / DNA replication origin binding / DNA replication initiation / subtelomeric heterochromatin formation / nucleosome binding / DNA helicase activity / transcription elongation by RNA polymerase II / G1/S transition of mitotic cell cycle / helicase activity / heterochromatin formation / single-stranded DNA binding / chromosome / DNA helicase / DNA replication / chromosome, telomeric region / cell division / GTPase activity / DNA damage response / chromatin binding / GTP binding / ATP hydrolysis activity / zinc ion binding / nucleoplasm / ATP binding / metal ion binding / nucleus / cytoplasm Similarity search - Function | |||||||||
| Biological species |   | |||||||||
| Method | single particle reconstruction / cryo EM / Resolution: 3.9 Å | |||||||||
 Authors Authors | Yuan Z / Riera A / Bai L / Sun J / Spanos C / Chen ZA / Barbon M / Rappsilber J / Stillman B / Speck C / Li H | |||||||||
 Citation Citation |  Journal: Nat Struct Mol Biol / Year: 2017 Journal: Nat Struct Mol Biol / Year: 2017Title: Structural basis of Mcm2-7 replicative helicase loading by ORC-Cdc6 and Cdt1. Authors: Zuanning Yuan / Alberto Riera / Lin Bai / Jingchuan Sun / Saikat Nandi / Christos Spanos / Zhuo Angel Chen / Marta Barbon / Juri Rappsilber / Bruce Stillman / Christian Speck / Huilin Li /    Abstract: To initiate DNA replication, the origin recognition complex (ORC) and Cdc6 load an Mcm2-7 double hexamer onto DNA. Without ATP hydrolysis, ORC-Cdc6 recruits one Cdt1-bound Mcm2-7 hexamer, thus ...To initiate DNA replication, the origin recognition complex (ORC) and Cdc6 load an Mcm2-7 double hexamer onto DNA. Without ATP hydrolysis, ORC-Cdc6 recruits one Cdt1-bound Mcm2-7 hexamer, thus forming an ORC-Cdc6-Cdt1-Mcm2-7 (OCCM) helicase-loading intermediate. Here we report a 3.9-Å structure of Saccharomyces cerevisiae OCCM on DNA. Flexible Mcm2-7 winged-helix domains (WHDs) engage ORC-Cdc6. A three-domain Cdt1 configuration embraces Mcm2, Mcm4, and Mcm6, thus comprising nearly half of the hexamer. The Cdt1 C-terminal domain extends to the Mcm6 WHD, which binds the Orc4 WHD. DNA passes through the ORC-Cdc6 and Mcm2-7 rings. Origin DNA interaction is mediated by an α-helix within Orc4 and positively charged loops within Orc2 and Cdc6. The Mcm2-7 C-tier AAA+ ring is topologically closed by an Mcm5 loop that embraces Mcm2, but the N-tier-ring Mcm2-Mcm5 interface remains open. This structure suggests a loading mechanism of the first Cdt1-bound Mcm2-7 hexamer by ORC-Cdc6. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Movie |
 Movie viewer Movie viewer |
|---|---|
| Structure viewer | EM map:  SurfView SurfView Molmil Molmil Jmol/JSmol Jmol/JSmol |
| Supplemental images |
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_8540.map.gz emd_8540.map.gz | 58.9 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-8540-v30.xml emd-8540-v30.xml emd-8540.xml emd-8540.xml | 35.8 KB 35.8 KB | Display Display |  EMDB header EMDB header |
| Images |  emd_8540.png emd_8540.png | 152.6 KB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-8540 http://ftp.pdbj.org/pub/emdb/structures/EMD-8540 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-8540 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-8540 | HTTPS FTP |
-Related structure data
| Related structure data |  5v8fMC  5udb M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_8540.map.gz / Format: CCP4 / Size: 64 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_8540.map.gz / Format: CCP4 / Size: 64 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Tetrameric HIV-1 Strand Transfer Complex Intasome | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 1.01 Å | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Density |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
CCP4 map header:
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
-Supplemental data
- Sample components
Sample components
+Entire : ORC-Cdc6-Cdt1-MCM2-7
+Supramolecule #1: ORC-Cdc6-Cdt1-MCM2-7
+Macromolecule #1: DNA replication licensing factor MCM2
+Macromolecule #2: DNA replication licensing factor MCM3
+Macromolecule #3: DNA replication licensing factor MCM4
+Macromolecule #4: Minichromosome maintenance protein 5
+Macromolecule #5: DNA replication licensing factor MCM6
+Macromolecule #6: DNA replication licensing factor MCM7
+Macromolecule #7: Cell division cycle protein CDT1
+Macromolecule #8: Cell division control protein 6
+Macromolecule #9: Origin recognition complex subunit 1
+Macromolecule #10: Origin recognition complex subunit 2
+Macromolecule #11: Origin recognition complex subunit 3
+Macromolecule #12: Origin recognition complex subunit 4
+Macromolecule #13: Origin recognition complex subunit 5
+Macromolecule #14: Origin recognition complex subunit 6
+Macromolecule #15: DNA (39-MER)
+Macromolecule #16: DNA (39-MER)
+Macromolecule #17: PHOSPHOTHIOPHOSPHORIC ACID-ADENYLATE ESTER
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Buffer | pH: 7.5 |
|---|---|
| Vitrification | Cryogen name: ETHANE |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Image recording | Film or detector model: GATAN K2 SUMMIT (4k x 4k) / Average electron dose: 50.0 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
- Image processing
Image processing
| Final reconstruction | Resolution.type: BY AUTHOR / Resolution: 3.9 Å / Resolution method: FSC 0.143 CUT-OFF / Number images used: 304288 |
|---|---|
| Initial angle assignment | Type: PROJECTION MATCHING |
| Final angle assignment | Type: PROJECTION MATCHING |
-Atomic model buiding 1
| Refinement | Protocol: RIGID BODY FIT |
|---|---|
| Output model |  PDB-5v8f: |
 Movie
Movie Controller
Controller















 X (Sec.)
X (Sec.) Y (Row.)
Y (Row.) Z (Col.)
Z (Col.)






















