+ Open data
Open data
- Basic information
Basic information
| Entry | Database: EMDB / ID: EMD-8177 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | Near atomic structure of the Dark apoptosome | |||||||||
 Map data Map data | Near atomic structure of the Dark apoptosome | |||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | Dark / apoptosome / apotosis / AAA+ ATPase / APOPTOSIS | |||||||||
| Function / homology |  Function and homology information Function and homology informationnegative regulation of humoral immune response / positive regulation of glial cell apoptotic process / Formation of apoptosome / salivary gland histolysis / melanization defense response / sarcosine catabolic process / Activation of caspases through apoptosome-mediated cleavage / Regulation of the apoptosome activity / compound eye retinal cell programmed cell death / central nervous system formation ...negative regulation of humoral immune response / positive regulation of glial cell apoptotic process / Formation of apoptosome / salivary gland histolysis / melanization defense response / sarcosine catabolic process / Activation of caspases through apoptosome-mediated cleavage / Regulation of the apoptosome activity / compound eye retinal cell programmed cell death / central nervous system formation / positive regulation of apoptotic process involved in development / chaeta development / sperm individualization / apoptosome / autophagic cell death / Neutrophil degranulation / S-adenosylmethionine cycle / CARD domain binding / programmed cell death / triglyceride homeostasis / response to starvation / dendrite morphogenesis / response to gamma radiation / neuron cellular homeostasis / ADP binding / apoptotic process / structural molecule activity / ATP binding / identical protein binding Similarity search - Function | |||||||||
| Biological species |  | |||||||||
| Method | single particle reconstruction / cryo EM / Resolution: 4.4 Å | |||||||||
 Authors Authors | Cheng TC / Akey IV | |||||||||
| Funding support |  United States, 1 items United States, 1 items
| |||||||||
 Citation Citation |  Journal: Structure / Year: 2017 Journal: Structure / Year: 2017Title: A Near-Atomic Structure of the Dark Apoptosome Provides Insight into Assembly and Activation. Authors: Tat Cheung Cheng / Ildikó V Akey / Shujun Yuan / Zhiheng Yu / Steven J Ludtke / Christopher W Akey /  Abstract: In Drosophila, the Apaf-1-related killer (Dark) forms an apoptosome that activates procaspases. To investigate function, we have determined a near-atomic structure of Dark double rings using cryo- ...In Drosophila, the Apaf-1-related killer (Dark) forms an apoptosome that activates procaspases. To investigate function, we have determined a near-atomic structure of Dark double rings using cryo-electron microscopy. We then built a nearly complete model of the apoptosome that includes 7- and 8-blade β-propellers. We find that the preference for dATP during Dark assembly may be governed by Ser325, which is in close proximity to the 2' carbon of the deoxyribose ring. Interestingly, β-propellers in V-shaped domains of the Dark apoptosome are more widely separated, relative to these features in the Apaf-1 apoptosome. This wider spacing may be responsible for the lack of cytochrome c binding to β-propellers in the Dark apoptosome. Our structure also highlights the roles of two loss-of-function mutations that may block Dark assembly. Finally, the improved model provides a framework to understand apical procaspase activation in the intrinsic cell death pathway. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Movie |
 Movie viewer Movie viewer |
|---|---|
| Structure viewer | EM map:  SurfView SurfView Molmil Molmil Jmol/JSmol Jmol/JSmol |
| Supplemental images |
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_8177.map.gz emd_8177.map.gz | 11.5 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-8177-v30.xml emd-8177-v30.xml emd-8177.xml emd-8177.xml | 22.1 KB 22.1 KB | Display Display |  EMDB header EMDB header |
| Images |  emd_8177.png emd_8177.png | 80.6 KB | ||
| Filedesc metadata |  emd-8177.cif.gz emd-8177.cif.gz | 7.9 KB | ||
| Others |  emd_8177_additional.map.gz emd_8177_additional.map.gz | 14.1 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-8177 http://ftp.pdbj.org/pub/emdb/structures/EMD-8177 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-8177 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-8177 | HTTPS FTP |
-Validation report
| Summary document |  emd_8177_validation.pdf.gz emd_8177_validation.pdf.gz | 428.9 KB | Display |  EMDB validaton report EMDB validaton report |
|---|---|---|---|---|
| Full document |  emd_8177_full_validation.pdf.gz emd_8177_full_validation.pdf.gz | 428.5 KB | Display | |
| Data in XML |  emd_8177_validation.xml.gz emd_8177_validation.xml.gz | 6.4 KB | Display | |
| Data in CIF |  emd_8177_validation.cif.gz emd_8177_validation.cif.gz | 7.4 KB | Display | |
| Arichive directory |  https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-8177 https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-8177 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-8177 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-8177 | HTTPS FTP |
-Related structure data
| Related structure data |  5julMC M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_8177.map.gz / Format: CCP4 / Size: 125 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_8177.map.gz / Format: CCP4 / Size: 125 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Near atomic structure of the Dark apoptosome | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 1.35 Å | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
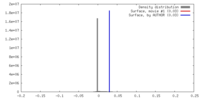
| Density |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
CCP4 map header:
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
-Supplemental data
-Additional map: Near atomic structure of the Dark apoptosome
| File | emd_8177_additional.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Near atomic structure of the Dark apoptosome | ||||||||||||
| Projections & Slices |
| ||||||||||||
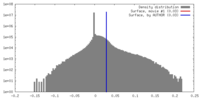
| Density Histograms |
- Sample components
Sample components
-Entire : Dark apoptosome
| Entire | Name: Dark apoptosome |
|---|---|
| Components |
|
-Supramolecule #1: Dark apoptosome
| Supramolecule | Name: Dark apoptosome / type: complex / ID: 1 / Parent: 0 / Macromolecule list: #1 |
|---|---|
| Source (natural) | Organism:  |
| Molecular weight | Theoretical: 2.3 MDa |
-Macromolecule #1: Apaf-1 related killer DARK
| Macromolecule | Name: Apaf-1 related killer DARK / type: protein_or_peptide / ID: 1 / Number of copies: 16 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  |
| Molecular weight | Theoretical: 166.131 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: MDFETGEHQY QYKDILSVFE DAFVDNFDCK DVQDMPKSIL SKEEIDHIIM SKDAVSGTLR LFWTLLSKQE EMVQKFVEEV LRINYKFLM SPIKTEQRQP SMMTRMYIEQ RDRLYNDNQV FAKYNVSRLQ PYLKLRQALL ELRPAKNVLI DGVLGSGKTW V ALDVCLSY ...String: MDFETGEHQY QYKDILSVFE DAFVDNFDCK DVQDMPKSIL SKEEIDHIIM SKDAVSGTLR LFWTLLSKQE EMVQKFVEEV LRINYKFLM SPIKTEQRQP SMMTRMYIEQ RDRLYNDNQV FAKYNVSRLQ PYLKLRQALL ELRPAKNVLI DGVLGSGKTW V ALDVCLSY KVQCKMDFKI FWLNLKNCNS PETVLEMLQK LLYQIDPNWT SRSDHSSNIK LRIHSIQAEL RRLLKSKPYE NC LLVLLNV QNA(APK)AWNAFN LSCKILLTTR FKQVTDFLSA ATTTHISLDH HSMTLTPDEV KSLLLKYLDC RPQDLPREV LTTNPRRLSI IAESIRDGLA TWDNWKHVNC DKLTTIIESS LNVLEPAEYR KMFDRLSVFP PSAHIPTILL SLIWFDVIKS DVMVVVNKL HKYSLVEKQP KESTISIPSI YLELKVKLEN EYALHRSIVD HYNIPKTFDS DDLIPPYLDQ YFYSHIGHHL K NIEHPERM TLFRMVFLDF RFLEQKIRHD STAWNASGSI LNTLQQLKFY KPYICDNDPK YERLVNAILD FLPKIEENLI CS KYTDLLR IALMAEDEAI FEEAHKQVQR FDDRVWFTNH GRFHQHRQII NLGDNEGRHA VYLHNDFCLI ALASGQILLT DVS LEGEDT YLLRDESDSS DILRMAVFNQ QKHLITLHCN GSVKLWSLWP DCPGRRHSGG SKQQLVNSVV KRFIGSYANL KIVA FYLNE DAGLPEANIQ LHVAFINGDV SILNWDEQDQ EFKLSHVPVL KTMQSGIRCF VQVLKRYYVV CTSNCTLTVW DLTNG SSNT LELHVFNVEN DTPLALDVFD ERSKTATVLL IFKYSVWRLN FLPGLSVSLQ SEAVQLPEGS FITCGKRSTD GRYLLL GTS EGLIVYDLKI SDPVLRSNVS EHIECVDIYE LFDPVYKYIV LCGAKGKQVV HVHTLRSVSG SNSHQNREIA WVHSADE IS VMTKACLEPN VYLRSLMDMT RERTQLLAVD SKERIHLIKP AISRISEWST ITPTHAASNC KINAISAFND EQIFVGYV D GVIIDVIHDT ALPQQFIEEP IDYLKQVSPN ILVASAHSAQ KTVIFQLEKI DPLQPNDQWP LMMDVSTKYA SLQEGQYII LFSDHGVCHL DIANPSAFVK PKDSEEYIVG FDLKNSLLFL AYENNIIDVF RLIFSCNQLR YEQICEEEIA QKAKISYLVA TDDGTMLAM GFENGTLELF AVENRKVQLI YSIEEVHEHC IRQLLFSPCK LLLISCAEQL CFWNVTHMRN NQLEREQKRR R SRRHKQHS VTQEDAVDAA PIAADIDVDV TFVADEFHPV NRGTAELWRN KRGNAIRPEL LACVKFVGNE ARQFFTDAHF SH FYAIDDE GVYYHLQLLE LSRLQPPPDP VTLDIANQYE DLKNLRILDS PLMQDSDSEG ADVVGNLVLE KNGGVARATP ILE EASS UniProtKB: Apoptosome protein Dark |
-Macromolecule #2: 2'-DEOXYADENOSINE 5'-TRIPHOSPHATE
| Macromolecule | Name: 2'-DEOXYADENOSINE 5'-TRIPHOSPHATE / type: ligand / ID: 2 / Number of copies: 16 / Formula: DTP |
|---|---|
| Molecular weight | Theoretical: 491.182 Da |
| Chemical component information |  ChemComp-DTP: |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Concentration | 2.0 mg/mL | ||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Buffer | pH: 7.5 Component:
Details: Buffer A | ||||||||||||||||||
| Grid | Model: C-flat-1.2/1.3 / Material: COPPER / Mesh: 300 / Support film - Material: CARBON / Support film - topology: HOLEY / Pretreatment - Type: GLOW DISCHARGE / Pretreatment - Time: 30 sec. | ||||||||||||||||||
| Vitrification | Cryogen name: ETHANE / Chamber humidity: 100 % / Chamber temperature: 293 K / Instrument: FEI VITROBOT MARK III Details: 2.5ul sample blot additives present at the indicated final concentrations: NP-40 (0.025%), DHPG (diheptanoylphosphatidylglycerol; 0.05%), cytochrome c (0.5 mg/ml), DeoxybigChaps (0.01%), and ...Details: 2.5ul sample blot additives present at the indicated final concentrations: NP-40 (0.025%), DHPG (diheptanoylphosphatidylglycerol; 0.05%), cytochrome c (0.5 mg/ml), DeoxybigChaps (0.01%), and lysine (0.03 mg/ml). |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Specialist optics | Spherical aberration corrector: FEI Cs corrector / Energy filter - Name: GIF |
| Image recording | Film or detector model: GATAN K2 SUMMIT (4k x 4k) / Detector mode: COUNTING / Digitization - Dimensions - Width: 7476 pixel / Digitization - Dimensions - Height: 7420 pixel / Digitization - Frames/image: 2-23 / Number grids imaged: 1 / Number real images: 1991 / Average exposure time: 0.3 sec. / Average electron dose: 1.8 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | C2 aperture diameter: 100.0 µm / Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD / Cs: 0.01 mm / Nominal defocus max: 2.4 µm / Nominal defocus min: 1.5 µm / Nominal magnification: 81000 |
| Sample stage | Specimen holder model: FEI TITAN KRIOS AUTOGRID HOLDER / Cooling holder cryogen: NITROGEN |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
+ Image processing
Image processing
-Atomic model buiding 1
| Details | local fitting with Chimera followed by flexible fitting with MDFF and refinement with Phenix. |
|---|---|
| Refinement | Protocol: FLEXIBLE FIT |
| Output model |  PDB-5jul: |
 Movie
Movie Controller
Controller












 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)