+ データを開く
データを開く
- 基本情報
基本情報
| 登録情報 | データベース: EMDB / ID: EMD-7771 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
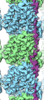
| タイトル | Cryo-EM reconstruction of microtubule-bound synthetic (R2x4) tau | |||||||||
 マップデータ マップデータ | Cryo-EM reconstruction of microtubule-bound synthetic tau (R2x4) | |||||||||
 試料 試料 |
| |||||||||
 キーワード キーワード | microtubule / tau / STRUCTURAL PROTEIN | |||||||||
| 機能・相同性 |  機能・相同性情報 機能・相同性情報Microtubule-dependent trafficking of connexons from Golgi to the plasma membrane / Resolution of Sister Chromatid Cohesion / Hedgehog 'off' state / Cilium Assembly / Intraflagellar transport / COPI-dependent Golgi-to-ER retrograde traffic / Mitotic Prometaphase / Carboxyterminal post-translational modifications of tubulin / RHOH GTPase cycle / EML4 and NUDC in mitotic spindle formation ...Microtubule-dependent trafficking of connexons from Golgi to the plasma membrane / Resolution of Sister Chromatid Cohesion / Hedgehog 'off' state / Cilium Assembly / Intraflagellar transport / COPI-dependent Golgi-to-ER retrograde traffic / Mitotic Prometaphase / Carboxyterminal post-translational modifications of tubulin / RHOH GTPase cycle / EML4 and NUDC in mitotic spindle formation / Sealing of the nuclear envelope (NE) by ESCRT-III / Kinesins / PKR-mediated signaling / Separation of Sister Chromatids / The role of GTSE1 in G2/M progression after G2 checkpoint / Aggrephagy / RHO GTPases activate IQGAPs / RHO GTPases Activate Formins / HSP90 chaperone cycle for steroid hormone receptors (SHR) in the presence of ligand / MHC class II antigen presentation / Recruitment of NuMA to mitotic centrosomes / COPI-mediated anterograde transport / plus-end-directed organelle transport along microtubule / histone-dependent DNA binding / negative regulation of establishment of protein localization to mitochondrion / neurofibrillary tangle / microtubule lateral binding / axonal transport / tubulin complex / positive regulation of protein localization to synapse / negative regulation of tubulin deacetylation / phosphatidylinositol bisphosphate binding / generation of neurons / rRNA metabolic process / axonal transport of mitochondrion / regulation of mitochondrial fission / axon development / regulation of chromosome organization / central nervous system neuron development / intracellular distribution of mitochondria / minor groove of adenine-thymine-rich DNA binding / lipoprotein particle binding / microtubule polymerization / negative regulation of mitochondrial membrane potential / dynactin binding / regulation of microtubule polymerization / apolipoprotein binding / main axon / protein polymerization / axolemma / glial cell projection / Caspase-mediated cleavage of cytoskeletal proteins / regulation of microtubule polymerization or depolymerization / negative regulation of mitochondrial fission / neurofibrillary tangle assembly / positive regulation of axon extension / regulation of cellular response to heat / Activation of AMPK downstream of NMDARs / synapse assembly / positive regulation of superoxide anion generation / regulation of long-term synaptic depression / positive regulation of protein localization / supramolecular fiber organization / cellular response to brain-derived neurotrophic factor stimulus / regulation of calcium-mediated signaling / cytoplasmic microtubule organization / positive regulation of microtubule polymerization / somatodendritic compartment / axon cytoplasm / astrocyte activation / stress granule assembly / phosphatidylinositol binding / nuclear periphery / regulation of microtubule cytoskeleton organization / protein phosphatase 2A binding / cellular response to reactive oxygen species / Hsp90 protein binding / microglial cell activation / cellular response to nerve growth factor stimulus / synapse organization / protein homooligomerization / PKR-mediated signaling / regulation of synaptic plasticity / SH3 domain binding / structural constituent of cytoskeleton / response to lead ion / microtubule cytoskeleton organization / memory / cytoplasmic ribonucleoprotein granule / neuron migration / neuron projection development / cell-cell signaling / mitotic cell cycle / single-stranded DNA binding / protein-folding chaperone binding / cellular response to heat / actin binding / microtubule cytoskeleton / cell body / growth cone 類似検索 - 分子機能 | |||||||||
| 生物種 |   Homo sapiens (ヒト) Homo sapiens (ヒト) | |||||||||
| 手法 | らせん対称体再構成法 / クライオ電子顕微鏡法 / 解像度: 3.9 Å | |||||||||
 データ登録者 データ登録者 | Nogales E / Kellogg EH | |||||||||
| 資金援助 |  米国, 1件 米国, 1件
| |||||||||
 引用 引用 |  ジャーナル: Science / 年: 2018 ジャーナル: Science / 年: 2018タイトル: Near-atomic model of microtubule-tau interactions. 著者: Elizabeth H Kellogg / Nisreen M A Hejab / Simon Poepsel / Kenneth H Downing / Frank DiMaio / Eva Nogales /  要旨: Tau is a developmentally regulated axonal protein that stabilizes and bundles microtubules (MTs). Its hyperphosphorylation is thought to cause detachment from MTs and subsequent aggregation into ...Tau is a developmentally regulated axonal protein that stabilizes and bundles microtubules (MTs). Its hyperphosphorylation is thought to cause detachment from MTs and subsequent aggregation into fibrils implicated in Alzheimer's disease. It is unclear which tau residues are crucial for tau-MT interactions, where tau binds on MTs, and how it stabilizes them. We used cryo-electron microscopy to visualize different tau constructs on MTs and computational approaches to generate atomic models of tau-tubulin interactions. The conserved tubulin-binding repeats within tau adopt similar extended structures along the crest of the protofilament, stabilizing the interface between tubulin dimers. Our structures explain the effect of phosphorylation on MT affinity and lead to a model of tau repeats binding in tandem along protofilaments, tethering together tubulin dimers and stabilizing polymerization interfaces. | |||||||||
| 履歴 |
|
- 構造の表示
構造の表示
| ムービー |
 ムービービューア ムービービューア |
|---|---|
| 構造ビューア | EMマップ:  SurfView SurfView Molmil Molmil Jmol/JSmol Jmol/JSmol |
| 添付画像 |
- ダウンロードとリンク
ダウンロードとリンク
-EMDBアーカイブ
| マップデータ |  emd_7771.map.gz emd_7771.map.gz | 278.3 MB |  EMDBマップデータ形式 EMDBマップデータ形式 | |
|---|---|---|---|---|
| ヘッダ (付随情報) |  emd-7771-v30.xml emd-7771-v30.xml emd-7771.xml emd-7771.xml | 19.7 KB 19.7 KB | 表示 表示 |  EMDBヘッダ EMDBヘッダ |
| 画像 |  emd_7771.png emd_7771.png | 152.3 KB | ||
| Filedesc metadata |  emd-7771.cif.gz emd-7771.cif.gz | 7.1 KB | ||
| アーカイブディレクトリ |  http://ftp.pdbj.org/pub/emdb/structures/EMD-7771 http://ftp.pdbj.org/pub/emdb/structures/EMD-7771 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-7771 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-7771 | HTTPS FTP |
-検証レポート
| 文書・要旨 |  emd_7771_validation.pdf.gz emd_7771_validation.pdf.gz | 645.7 KB | 表示 |  EMDB検証レポート EMDB検証レポート |
|---|---|---|---|---|
| 文書・詳細版 |  emd_7771_full_validation.pdf.gz emd_7771_full_validation.pdf.gz | 645.2 KB | 表示 | |
| XML形式データ |  emd_7771_validation.xml.gz emd_7771_validation.xml.gz | 7.4 KB | 表示 | |
| CIF形式データ |  emd_7771_validation.cif.gz emd_7771_validation.cif.gz | 8.5 KB | 表示 | |
| アーカイブディレクトリ |  https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-7771 https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-7771 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-7771 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-7771 | HTTPS FTP |
-関連構造データ
- リンク
リンク
| EMDBのページ |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| 「今月の分子」の関連する項目 |
- マップ
マップ
| ファイル |  ダウンロード / ファイル: emd_7771.map.gz / 形式: CCP4 / 大きさ: 307.5 MB / タイプ: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) ダウンロード / ファイル: emd_7771.map.gz / 形式: CCP4 / 大きさ: 307.5 MB / タイプ: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 注釈 | Cryo-EM reconstruction of microtubule-bound synthetic tau (R2x4) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 投影像・断面図 | 画像のコントロール
画像は Spider により作成 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| ボクセルのサイズ | X=Y=Z: 1.32 Å | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
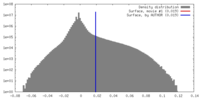
| 密度 |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 対称性 | 空間群: 1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 詳細 | EMDB XML:
CCP4マップ ヘッダ情報:
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
-添付データ
- 試料の構成要素
試料の構成要素
+全体 : Ternary complex of alpha-beta tubulin with synthetic (R2x4) tau
+超分子 #1: Ternary complex of alpha-beta tubulin with synthetic (R2x4) tau
+超分子 #2: Tubulin beta chain
+超分子 #3: Tubulin alpha-1B chain
+超分子 #4: Microtubule-associated protein tau
+分子 #1: Tubulin beta chain
+分子 #2: Tubulin alpha-1B chain
+分子 #3: Microtubule-associated protein tau
+分子 #4: GUANOSINE-5'-DIPHOSPHATE
+分子 #5: GUANOSINE-5'-TRIPHOSPHATE
+分子 #6: MAGNESIUM ION
-実験情報
-構造解析
| 手法 | クライオ電子顕微鏡法 |
|---|---|
 解析 解析 | らせん対称体再構成法 |
| 試料の集合状態 | filament |
- 試料調製
試料調製
| 濃度 | 0.5 mg/mL | ||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 緩衝液 | pH: 6.8 構成要素:
| ||||||||||||||||||
| グリッド | モデル: C-flat-1.2/1.3 4C / 材質: COPPER / メッシュ: 400 / 支持フィルム - 材質: CARBON / 支持フィルム - トポロジー: HOLEY / 前処理 - タイプ: GLOW DISCHARGE / 前処理 - 時間: 10 sec. / 前処理 - 雰囲気: AIR | ||||||||||||||||||
| 凍結 | 凍結剤: ETHANE / チャンバー内湿度: 100 % / チャンバー内温度: 310.15 K / 装置: FEI VITROBOT MARK IV 詳細: blot force 10 pN, 6 second blot time. used C-flat 1.2/1.3 holey grids. First 2 uL of microtubules were adhered to grid for 30 seconds, followed by 2 4 uL washes of tau with 30 second incubation for each wash.. | ||||||||||||||||||
| 詳細 | tubulin concentration is 0.5 mg/mL, tau concentration is 1 mg/mL |
- 電子顕微鏡法
電子顕微鏡法
| 顕微鏡 | FEI TITAN KRIOS |
|---|---|
| 特殊光学系 | 位相板: VOLTA PHASE PLATE |
| 撮影 | フィルム・検出器のモデル: GATAN K2 BASE (4k x 4k) 撮影したグリッド数: 1 / 実像数: 812 / 平均露光時間: 10.0 sec. / 平均電子線量: 40.5 e/Å2 |
| 電子線 | 加速電圧: 300 kV / 電子線源:  FIELD EMISSION GUN FIELD EMISSION GUN |
| 電子光学系 | C2レンズ絞り径: 100.0 µm / 照射モード: FLOOD BEAM / 撮影モード: BRIGHT FIELD / Cs: 2.7 mm / 最小 デフォーカス(公称値): 0.5 µm / 倍率(公称値): 37878 |
| 試料ステージ | 試料ホルダーモデル: FEI TITAN KRIOS AUTOGRID HOLDER |
| 実験機器 |  モデル: Titan Krios / 画像提供: FEI Company |
- 画像解析
画像解析
| 最終 再構成 | 想定した対称性 - らせんパラメータ - Δz: 8.7 Å 想定した対称性 - らせんパラメータ - ΔΦ: -25.75 ° 想定した対称性 - らせんパラメータ - 軸対称性: C1 (非対称) アルゴリズム: FOURIER SPACE / 解像度のタイプ: BY AUTHOR / 解像度: 3.9 Å / 解像度の算出法: FSC 0.143 CUT-OFF / ソフトウェア - 名称: FREALIGN (ver. 9.09) / 使用した粒子像数: 19505 |
|---|---|
| Segment selection | 選択した数: 24571 / ソフトウェア - 名称: Appion |
| 初期モデル | モデルのタイプ: EMDB MAP EMDB ID: 詳細: used naked microtubule reconstruction as initial model, low-pass filtered to 20 Angstrom |
| 最終 角度割当 | タイプ: NOT APPLICABLE / ソフトウェア - 名称: FREALIGN (ver. 9.09) |
-原子モデル構築 1
| 精密化 | 空間: REAL / プロトコル: FLEXIBLE FIT / 温度因子: 120 / 当てはまり具合の基準: real space correlation |
|---|---|
| 得られたモデル |  PDB-6cvn: |
 ムービー
ムービー コントローラー
コントローラー



















 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)