[English] 日本語
 Yorodumi
Yorodumi- EMDB-6996: Complex of voltage-gated sodium channel NavPaS from American cock... -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: EMDB / ID: EMD-6996 | ||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Title | Complex of voltage-gated sodium channel NavPaS from American cockroach Periplaneta americana bound with saxitoxin and Dc1a | ||||||||||||||||||||||||
 Map data Map data | complex of voltage-gated sodium channel NavPaS from American cockroach Periplaneta americana bound with saxitoxin and Dc1a | ||||||||||||||||||||||||
 Sample Sample |
| ||||||||||||||||||||||||
 Keywords Keywords | complex / sodium channel / toxin / MEMBRANE PROTEIN / MEMBRANE PROTEIN-TOXIN complex | ||||||||||||||||||||||||
| Function / homology |  Function and homology information Function and homology informationmembrane depolarization during action potential / voltage-gated sodium channel complex / host cell presynaptic membrane / voltage-gated sodium channel activity / voltage-gated monoatomic cation channel activity / sodium channel regulator activity / neuronal action potential / toxin activity / extracellular region Similarity search - Function | ||||||||||||||||||||||||
| Biological species |  Periplaneta americana (American cockroach) / Periplaneta americana (American cockroach) /  Diguetia canities (spider) Diguetia canities (spider) | ||||||||||||||||||||||||
| Method | single particle reconstruction / cryo EM / Resolution: 3.2 Å | ||||||||||||||||||||||||
 Authors Authors | Shen HZ / li ZQ | ||||||||||||||||||||||||
| Funding support |  China, China,  Australia, 7 items Australia, 7 items
| ||||||||||||||||||||||||
 Citation Citation |  Journal: Science / Year: 2018 Journal: Science / Year: 2018Title: Structural basis for the modulation of voltage-gated sodium channels by animal toxins. Authors: Huaizong Shen / Zhangqiang Li / Yan Jiang / Xiaojing Pan / Jianping Wu / Ben Cristofori-Armstrong / Jennifer J Smith / Yanni K Y Chin / Jianlin Lei / Qiang Zhou / Glenn F King / Nieng Yan /   Abstract: Animal toxins that modulate the activity of voltage-gated sodium (Na) channels are broadly divided into two categories-pore blockers and gating modifiers. The pore blockers tetrodotoxin (TTX) and ...Animal toxins that modulate the activity of voltage-gated sodium (Na) channels are broadly divided into two categories-pore blockers and gating modifiers. The pore blockers tetrodotoxin (TTX) and saxitoxin (STX) are responsible for puffer fish and shellfish poisoning in humans, respectively. Here, we present structures of the insect Na channel NaPaS bound to a gating modifier toxin Dc1a at 2.8 angstrom-resolution and in the presence of TTX or STX at 2.6-Å and 3.2-Å resolution, respectively. Dc1a inserts into the cleft between VSD and the pore of NaPaS, making key contacts with both domains. The structures with bound TTX or STX reveal the molecular details for the specific blockade of Na access to the selectivity filter from the extracellular side by these guanidinium toxins. The structures shed light on structure-based development of Na channel drugs. | ||||||||||||||||||||||||
| History |
|
- Structure visualization
Structure visualization
| Movie |
 Movie viewer Movie viewer |
|---|---|
| Structure viewer | EM map:  SurfView SurfView Molmil Molmil Jmol/JSmol Jmol/JSmol |
| Supplemental images |
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_6996.map.gz emd_6996.map.gz | 117.1 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-6996-v30.xml emd-6996-v30.xml emd-6996.xml emd-6996.xml | 23.9 KB 23.9 KB | Display Display |  EMDB header EMDB header |
| Images |  emd_6996.png emd_6996.png | 44.3 KB | ||
| Filedesc metadata |  emd-6996.cif.gz emd-6996.cif.gz | 8 KB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-6996 http://ftp.pdbj.org/pub/emdb/structures/EMD-6996 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-6996 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-6996 | HTTPS FTP |
-Related structure data
| Related structure data |  6a91MC  6995C  6997C  6a90C  6a95C M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_6996.map.gz / Format: CCP4 / Size: 125 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_6996.map.gz / Format: CCP4 / Size: 125 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | complex of voltage-gated sodium channel NavPaS from American cockroach Periplaneta americana bound with saxitoxin and Dc1a | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 1.091 Å | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
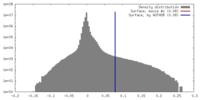
| Density |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
CCP4 map header:
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
-Supplemental data
- Sample components
Sample components
-Entire : complex of voltage-gated sodium channel NavPaS from American cock...
| Entire | Name: complex of voltage-gated sodium channel NavPaS from American cockroach Periplaneta americana bound with saxitoxin and Dc1a |
|---|---|
| Components |
|
-Supramolecule #1: complex of voltage-gated sodium channel NavPaS from American cock...
| Supramolecule | Name: complex of voltage-gated sodium channel NavPaS from American cockroach Periplaneta americana bound with saxitoxin and Dc1a type: complex / ID: 1 / Parent: 0 / Macromolecule list: #1-#2 |
|---|---|
| Molecular weight | Theoretical: 200 KDa |
-Supramolecule #2: voltage-gated sodium channel NavPaS from American cockroach Perip...
| Supramolecule | Name: voltage-gated sodium channel NavPaS from American cockroach Periplaneta americana type: complex / ID: 2 / Parent: 1 / Macromolecule list: #1 |
|---|---|
| Source (natural) | Organism:  Periplaneta americana (American cockroach) Periplaneta americana (American cockroach) |
-Supramolecule #3: Dc1a
| Supramolecule | Name: Dc1a / type: complex / ID: 3 / Parent: 1 / Macromolecule list: #2 |
|---|---|
| Source (natural) | Organism:  Diguetia canities (spider) Diguetia canities (spider) |
-Macromolecule #1: Sodium channel protein PaFPC1
| Macromolecule | Name: Sodium channel protein PaFPC1 / type: protein_or_peptide / ID: 1 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Periplaneta americana (American cockroach) Periplaneta americana (American cockroach) |
| Molecular weight | Theoretical: 183.875422 KDa |
| Recombinant expression | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Sequence | String: MASWSHPQFE KGGGARGGSG GGSWSHPQFE KGFDYKDDDD KGTMADNSPL IREERQRLFR PYTRAMLTAP SAQPAKENGK TEENKDNSR DKGRGANKDR DGSAHPDQAL EQGSRLPARM RNIFPAELAS TPLEDFDPFY KNKKTFVVVT KAGDIFRFSG E KSLWMLDP ...String: MASWSHPQFE KGGGARGGSG GGSWSHPQFE KGFDYKDDDD KGTMADNSPL IREERQRLFR PYTRAMLTAP SAQPAKENGK TEENKDNSR DKGRGANKDR DGSAHPDQAL EQGSRLPARM RNIFPAELAS TPLEDFDPFY KNKKTFVVVT KAGDIFRFSG E KSLWMLDP FTPIRRVAIS TMVQPIFSYF IMITILIHCI FMIMPATQTT YILELVFLSI YTIEVVVKVL ARGFILHPFA YL RDPWNWL DFLVTLIGYI TLVVDLGHLY ALRAFRVLRS WRTVTIVPGW RTIVDALSLS ITSLKDLVLL LLFSLFVFAV LGL QIYMGV LTQKCVKHFP ADGSWGNFTD ERWFNYTSNS SHWYIPDDWI EYPLCGNSSG AGMCPPGYTC LQGYGGNPNY GYTS FDTFG WAFLSVFRLV TLDYWEDLYQ LALRSAGPWH ILFFIIVVFY GTFCFLNFIL AVVVMSYTHM VKRADEEKAA ERELK KEKK AASVANNTAN GQEQTTIEMN GDEAVVIDNN DQAARQQSDP ETPAPSVTQR LTDFLCVWDC CVPWQKLQGA IGAVVL SPF FELFIAVIIV LNITFMALDH HDMNIEFERI LRTGNYIFTS IYIVEAVLKI IALSPKFYFK DSWNVFDFII VVFAILE LG LEGVQGLSVF RSFRLLRVFR LAKFWPTLNN FMSVMTKSYG AFVNVMYVMF LLLFIFAIIG MQLFGMNYID NMERFPDG D LPRWNFTDFL HSFMIVFRAL CGEWIESMWD CMLVGDWSCI PFFVAVFFVG NLVILNLLIA LLLNNYGSFC TSPTSDEED SKDEDALAQI VRIFKRFKPN LNAVKLSPMK PDSEDIVESQ EIQGNNIADA EDVLAGEFPP DCCCNAFYKC FPSRPARDSS VQRMWSNIR RVCFLLAKNK YFQKFVTAVL VITSVLLALE DIYLPQRPVL VNITLYVDYV LTAFFVIEMI IMLFAVGFKK Y FTSKWYWL DFIVVVAYLL NFVLMCAGIE ALQTLRLLRV FRLFRPLSKV NGMQVVTSTL VEAVPHIFNV ILVGIFFWLV FA IMGVQLF AGKFYKCVDE NSTVLSHEIT MDRNDCLHEN YTWENSPMNF DHVGNAYLSL LQVATFKGWL QIMNDAIDSR EVH KQPIRE TNIYMYLYFI FFIVFGSFFI LKLFVCILID IFRQQRRKAE GLSATDSRTQ LIYRRAVMRT MSAKPVKRIP KPTC HPQSL MYDISVNRKF EYTMMILIIL NVAVMAIDHY GQSMEFSEVL DYLNLIFIII FFVECVIKVS GLRHHYFKDP WNIID FLYV VLAIAGLMLS DVIEKYFISP TLLRILRILR VGRLLRYFQS ARGMRLLLLA LRKALRTLFN VSFLLFVIMF VYAVFG MEF FMHIRDAGAI DDVYNFKTFG QSIILLFQLA TSAGWDGVYF AIANEEDCRA PDHELGYPGN CGSRALGIAY LVSYLII TC LVVINMYAAV ILDYVLEVYE DSKEGLTDDD YDMFFEVWQQ FDPEATQYIR YDQLSELLEA LQPPLQVQKP NKYKILSM N IPICKDDHIF YKDVLEALVK DVFSRRGSPV EAGDVQAPNV DEAEYKPVSS TLQRQREEYC VRLIQNAWRK HKQQN UniProtKB: Sodium channel protein PaFPC1 |
-Macromolecule #2: Mu-diguetoxin-Dc1a
| Macromolecule | Name: Mu-diguetoxin-Dc1a / type: protein_or_peptide / ID: 2 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Diguetia canities (spider) Diguetia canities (spider) |
| Molecular weight | Theoretical: 6.502441 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: SAKDGDVEGP AGCKKYDVEC DSGECCQKQY LWYKWRPLDC RCLKSGFFSS KCVCRDV UniProtKB: Beta-diguetoxin-Dc1a |
-Macromolecule #4: 2-acetamido-2-deoxy-beta-D-glucopyranose
| Macromolecule | Name: 2-acetamido-2-deoxy-beta-D-glucopyranose / type: ligand / ID: 4 / Number of copies: 3 / Formula: NAG |
|---|---|
| Molecular weight | Theoretical: 221.208 Da |
| Chemical component information |  ChemComp-NAG: |
-Macromolecule #5: 1,2-DIACYL-SN-GLYCERO-3-PHOSPHOCHOLINE
| Macromolecule | Name: 1,2-DIACYL-SN-GLYCERO-3-PHOSPHOCHOLINE / type: ligand / ID: 5 / Number of copies: 2 / Formula: PC1 |
|---|---|
| Molecular weight | Theoretical: 790.145 Da |
| Chemical component information |  ChemComp-PC1: |
-Macromolecule #6: [(3aS,4R,10aS)-2,6-diamino-10,10-dihydroxy-3a,4,9,10-tetrahydro-3...
| Macromolecule | Name: [(3aS,4R,10aS)-2,6-diamino-10,10-dihydroxy-3a,4,9,10-tetrahydro-3H,8H-pyrrolo[1,2-c]purin-4-yl]methyl carbamate type: ligand / ID: 6 / Number of copies: 1 / Formula: 9SL |
|---|---|
| Molecular weight | Theoretical: 299.286 Da |
| Chemical component information |  ChemComp-9SL: |
-Macromolecule #7: water
| Macromolecule | Name: water / type: ligand / ID: 7 / Number of copies: 3 / Formula: HOH |
|---|---|
| Molecular weight | Theoretical: 18.015 Da |
| Chemical component information |  ChemComp-HOH: |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Buffer | pH: 7.4 |
|---|---|
| Vitrification | Cryogen name: ETHANE |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Image recording | Film or detector model: GATAN K2 QUANTUM (4k x 4k) / Average electron dose: 48.0 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
 Movie
Movie Controller
Controller










 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)