+ Open data
Open data
- Basic information
Basic information
| Entry | Database: EMDB / ID: EMD-6862 | ||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
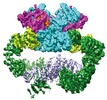
| Title | Cryo-EM Structure of human ATR-ATRIP complex | ||||||||||||||||||||||||
 Map data Map data | |||||||||||||||||||||||||
 Sample Sample |
| ||||||||||||||||||||||||
 Keywords Keywords | cryo-EM / ATR-ATRIP / DNA damnage response / CELL CYCLE | ||||||||||||||||||||||||
| Function / homology |  Function and homology information Function and homology informationATR-ATRIP complex / establishment of RNA localization to telomere / positive regulation of telomerase catalytic core complex assembly / establishment of protein-containing complex localization to telomere / MutSalpha complex binding / histone H2AXS139 kinase activity / nuclear membrane disassembly / MutLalpha complex binding / response to arsenic-containing substance / mitotic G2/M transition checkpoint ...ATR-ATRIP complex / establishment of RNA localization to telomere / positive regulation of telomerase catalytic core complex assembly / establishment of protein-containing complex localization to telomere / MutSalpha complex binding / histone H2AXS139 kinase activity / nuclear membrane disassembly / MutLalpha complex binding / response to arsenic-containing substance / mitotic G2/M transition checkpoint / regulation of double-strand break repair / nucleobase-containing compound metabolic process / positive regulation of DNA damage response, signal transduction by p53 class mediator / protein localization to chromosome, telomeric region / K63-linked polyubiquitin modification-dependent protein binding / HDR through Single Strand Annealing (SSA) / Impaired BRCA2 binding to RAD51 / negative regulation of DNA replication / replication fork processing / Presynaptic phase of homologous DNA pairing and strand exchange / replicative senescence / Regulation of HSF1-mediated heat shock response / response to mechanical stimulus / site of DNA damage / interstrand cross-link repair / Activation of ATR in response to replication stress / regulation of cellular response to heat / positive regulation of telomere maintenance via telomerase / telomere maintenance / DNA damage checkpoint signaling / Meiotic synapsis / TP53 Regulates Transcription of DNA Repair Genes / Fanconi Anemia Pathway / cellular response to gamma radiation / PML body / G2/M DNA damage checkpoint / cellular response to UV / nuclear envelope / double-strand break repair / chromosome / Processing of DNA double-strand break ends / Regulation of TP53 Activity through Phosphorylation / DNA replication / protein kinase activity / non-specific serine/threonine protein kinase / response to xenobiotic stimulus / protein serine kinase activity / DNA repair / protein serine/threonine kinase activity / DNA damage response / Golgi apparatus / DNA binding / nucleoplasm / ATP binding / nucleus Similarity search - Function | ||||||||||||||||||||||||
| Biological species |  Homo sapiens (human) Homo sapiens (human) | ||||||||||||||||||||||||
| Method | single particle reconstruction / cryo EM / Resolution: 4.7 Å | ||||||||||||||||||||||||
 Authors Authors | Rao Q / Liu M | ||||||||||||||||||||||||
| Funding support |  China, 7 items China, 7 items
| ||||||||||||||||||||||||
 Citation Citation |  Journal: Cell Res / Year: 2018 Journal: Cell Res / Year: 2018Title: Cryo-EM structure of human ATR-ATRIP complex. Authors: Qinhui Rao / Mengjie Liu / Yuan Tian / Zihan Wu / Yuhan Hao / Lei Song / Zhaoyu Qin / Chen Ding / Hong-Wei Wang / Jiawei Wang / Yanhui Xu /  Abstract: ATR (ataxia telangiectasia-mutated and Rad3-related) protein kinase and ATRIP (ATR-interacting protein) form a complex and play a critical role in response to replication stress and DNA damage. Here, ...ATR (ataxia telangiectasia-mutated and Rad3-related) protein kinase and ATRIP (ATR-interacting protein) form a complex and play a critical role in response to replication stress and DNA damage. Here, we determined the cryo-electron microscopy (EM) structure of the human ATR-ATRIP complex at 4.7 Å resolution and built an atomic model of the C-terminal catalytic core of ATR (residues 1 521-2 644) at 3.9 Å resolution. The complex adopts a hollow "heart" shape, consisting of two ATR monomers in distinct conformations. The EM map for ATRIP reveals 14 HEAT repeats in an extended "S" shape. The conformational flexibility of ATR allows ATRIP to properly lock the N-termini of the two ATR monomers to favor ATR-ATRIP complex formation and functional diversity. The isolated "head-head" and "tail-tail" each adopts a pseudo 2-fold symmetry. The catalytic pockets face outward and substrate access is not restricted by inhibitory elements. Our studies provide a structural basis for understanding the assembly of the ATR-ATRIP complex and a framework for characterizing ATR-mediated DNA repair pathways. | ||||||||||||||||||||||||
| History |
|
- Structure visualization
Structure visualization
| Movie |
 Movie viewer Movie viewer |
|---|---|
| Structure viewer | EM map:  SurfView SurfView Molmil Molmil Jmol/JSmol Jmol/JSmol |
| Supplemental images |
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_6862.map.gz emd_6862.map.gz | 6.5 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-6862-v30.xml emd-6862-v30.xml emd-6862.xml emd-6862.xml | 15 KB 15 KB | Display Display |  EMDB header EMDB header |
| FSC (resolution estimation) |  emd_6862_fsc.xml emd_6862_fsc.xml | 8.4 KB | Display |  FSC data file FSC data file |
| Images |  emd_6862.png emd_6862.png | 225.9 KB | ||
| Filedesc metadata |  emd-6862.cif.gz emd-6862.cif.gz | 7.4 KB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-6862 http://ftp.pdbj.org/pub/emdb/structures/EMD-6862 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-6862 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-6862 | HTTPS FTP |
-Related structure data
| Related structure data |  5yz0MC  6863C M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_6862.map.gz / Format: CCP4 / Size: 52.7 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_6862.map.gz / Format: CCP4 / Size: 52.7 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 1.3 Å | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Density |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
CCP4 map header:
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
-Supplemental data
- Sample components
Sample components
-Entire : ATR-ATRIP complex
| Entire | Name: ATR-ATRIP complex |
|---|---|
| Components |
|
-Supramolecule #1: ATR-ATRIP complex
| Supramolecule | Name: ATR-ATRIP complex / type: complex / ID: 1 / Parent: 0 / Macromolecule list: all |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 700 kDa/nm |
-Macromolecule #1: Serine/threonine-protein kinase ATR
| Macromolecule | Name: Serine/threonine-protein kinase ATR / type: protein_or_peptide / ID: 1 / Number of copies: 2 / Enantiomer: LEVO / EC number: non-specific serine/threonine protein kinase |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 301.756781 KDa |
| Recombinant expression | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Sequence | String: MGEHGLELAS MIPALRELGS ATPEEYNTVV QKPRQILCQF IDRILTDVNV VAVELVKKTD SQPTSVMLLD FIQHIMKSSP LMFVNVSGS HEAKGSCIEF SNWIITRLLR IAATPSCHLL HKKICEVICS LLFLFKSKSP AIFGVLTKEL LQLFEDLVYL H RRNVMGHA ...String: MGEHGLELAS MIPALRELGS ATPEEYNTVV QKPRQILCQF IDRILTDVNV VAVELVKKTD SQPTSVMLLD FIQHIMKSSP LMFVNVSGS HEAKGSCIEF SNWIITRLLR IAATPSCHLL HKKICEVICS LLFLFKSKSP AIFGVLTKEL LQLFEDLVYL H RRNVMGHA VEWPVVMSRF LSQLDEHMGY LQSAPLQLMS MQNLEFIEVT LLMVLTRIIA IVFFRRQELL LWQIGCVLLE YG SPKIKSL AISFLTELFQ LGGLPAQPAS TFFSSFLELL KHLVEMDTDQ LKLYEEPLSK LIKTLFPFEA EAYRNIEPVY LNM LLEKLC VMFEDGVLMR LKSDLLKAAL CHLLQYFLKF VPAGYESALQ VRKVYVRNIC KALLDVLGIE VDAEYLLGPL YAAL KMESM EIIEEIQCQT QQENLSSNSD GISPKRRRLS SSLNPSKRAP KQTEEIKHVD MNQKSILWSA LKQKAESLQI SLEYS GLKN PVIEMLEGIA VVLQLTALCT VHCSHQNMNC RTFKDCQHKS KKKPSVVITW MSLDFYTKVL KSCRSLLESV QKLDLE ATI DKVVKIYDAL IYMQVNSSFE DHILEDLCGM LSLPWIYSHS DDGCLKLTTF AANLLTLSCR ISDSYSPQAQ SRCVFLL TL FPRRIFLEWR TAVYNWALQS SHEVIRASCV SGFFILLQQQ NSCNRVPKIL IDKVKDDSDI VKKEFASILG QLVCTLHG M FYLTSSLTEP FSEHGHVDLF CRNLKATSQH ECSSSQLKAS VCKPFLFLLK KKIPSPVKLA FIDNLHHLCK HLDFREDET DVKAVLGTLL NLMEDPDKDV RVAFSGNIKH ILESLDSEDG FIKELFVLRM KEAYTHAQIS RNNELKDTLI LTTGDIGRAA KGDLVPFAL LHLLHCLLSK SASVSGAAYT EIRALVAAKS VKLQSFFSQY KKPICQFLVE SLHSSQMTAL PNTPCQNADV R KQDVAHQR EMALNTLSEI ANVFDFPDLN RFLTRTLQVL LPDLAAKASP AASALIRTLG KQLNVNRREI LINNFKYIFS HL VCSCSKD ELERALHYLK NETEIELGSL LRQDFQGLHN ELLLRIGEHY QQVFNGLSIL ASFASSDDPY QGPRDIISPE LMA DYLQPK LLGILAFFNM QLLSSSVGIE DKKMALNSLM SLMKLMGPKH VSSVRVKMMT TLRTGLRFKD DFPELCCRAW DCFV RCLDH ACLGSLLSHV IVALLPLIHI QPKETAAIFH YLIIENRDAV QDFLHEIYFL PDHPELKKIK AVLQEYRKET SESTD LQTT LQLSMKAIQH ENVDVRIHAL TSLKETLYKN QEKLIKYATD SETVEPIISQ LVTVLLKGCQ DANSQARLLC GECLGE LGA IDPGRLDFST TETQGKDFTF VTGVEDSSFA YGLLMELTRA YLAYADNSRA QDSAAYAIQE LLSIYDCREM ETNGPGH QL WRRFPEHVRE ILEPHLNTRY KSSQKSTDWS GVKKPIYLSK LGSNFAEWSA SWAGYLITKV RHDLASKIFT CCSIMMKH D FKVTIYLLPH ILVYVLLGCN QEDQQEVYAE IMAVLKHDDQ HTINTQDIAS DLCQLSTQTV FSMLDHLTQW ARHKFQALK AEKCPHSKSN RNKVDSMVST VDYEDYQSVT RFLDLIPQDT LAVASFRSKA YTRAVMHFES FITEKKQNIQ EHLGFLQKLY AAMHEPDGV AGVSAIRKAE PSLKEQILEH ESLGLLRDAT ACYDRAIQLE PDQIIHYHGV VKSMLGLGQL STVITQVNGV H ANRSEWTD ELNTYRVEAA WKLSQWDLVE NYLAADGKST TWSVRLGQLL LSAKKRDITA FYDSLKLVRA EQIVPLSAAS FE RGSYQRG YEYIVRLHML CELEHSIKPL FQHSPGDSSQ EDSLNWVARL EMTQNSYRAK EPILALRRAL LSLNKRPDYN EMV GECWLQ SARVARKAGH HQTAYNALLN AGESRLAELY VERAKWLWSK GDVHQALIVL QKGVELCFPE NETPPEGKNM LIHG RAMLL VGRFMEETAN FESNAIMKKY KDVTACLPEW EDGHFYLAKY YDKLMPMVTD NKMEKQGDLI RYIVLHFGRS LQYGN QFIY QSMPRMLTLW LDYGTKAYEW EKAGRSDRVQ MRNDLGKINK VITEHTNYLA PYQFLTAFSQ LISRICHSHD EVFVVL MEI IAKVFLAYPQ QAMWMMTAVS KSSYPMRVNR CKEILNKAIH MKKSLEKFVG DATRLTDKLL ELCNKPVDGS SSTLSMS TH FKMLKKLVEE ATFSEILIPL QSVMIPTLPS ILGTHANHAS HEPFPGHWAY IAGFDDMVEI LASLQKPKKI SLKGSDGK F YIMMCKPKDD LRKDCRLMEF NSLINKCLRK DAESRRRELH IRTYAVIPLN DECGIIEWVN NTAGLRPILT KLYKEKGVY MTGKELRQCM LPKSAALSEK LKVFREFLLP RHPPIFHEWF LRTFPDPTSW YSSRSAYCRS TAVMSMVGYI LGLGDRHGEN ILFDSLTGE CVHVDFNCLF NKGETFEVPE IVPFRLTHNM VNGMGPMGTE GLFRRACEVT MRLMRDQREP LMSVLKTFLH D PLVEWSKP VKGHSKAPLN ETGEVVNEKA KTHVLDIEQR LQGVIKTRNR VTGLPLSIEG HVHYLIQEAT DENLLCQMYL GW TPYM UniProtKB: Serine/threonine-protein kinase ATR |
-Macromolecule #2: ATR-interacting protein
| Macromolecule | Name: ATR-interacting protein / type: protein_or_peptide / ID: 2 / Number of copies: 2 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 85.940664 KDa |
| Recombinant expression | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Sequence | String: MAGTSAPGSK RRSEPPAPRP GPPPGTGHPP SKRARGFSAA AAPDPDDPFG AHGDFTADDL EELDTLASQA LSQCPAAARD VSSDHKVHR LLDGMSKNPS GKNRETVPIK DNFELEVLQA QYKELKEKMK VMEEEVLIKN GEIKILRDSL HQTESVLEEQ R RSHFLLEQ ...String: MAGTSAPGSK RRSEPPAPRP GPPPGTGHPP SKRARGFSAA AAPDPDDPFG AHGDFTADDL EELDTLASQA LSQCPAAARD VSSDHKVHR LLDGMSKNPS GKNRETVPIK DNFELEVLQA QYKELKEKMK VMEEEVLIKN GEIKILRDSL HQTESVLEEQ R RSHFLLEQ EKTQALSDKE KEFSKKLQSL QSELQFKDAE MNELRTKLQT SERANKLAAP SVSHVSPRKN PSVVIKPEAC SP QFGKTSF PTKESFSANM SLPHPCQTES GYKPLVGRED SKPHSLRGDS IKQEEAQKSF VDSWRQRSNT QGSILINLLL KQP LIPGSS LSLCHLLSSS SESPAGTPLQ PPGFGSTLAG MSGLRTTGSY DGSFSLSALR EAQNLAFTGL NLVARNECSR DGDP AEGGR RAFPLCQLPG AVHFLPLVQF FIGLHCQALQ DLAAAKRSGA PGDSPTHSSC VSSGVETNPE DSVCILEGFS VTALS ILQH LVCHSGAVVS LLLSGVGADS AAGEGNRSLV HRLSDGDMTS ALRGVADDQG QHPLLKMLLH LLAFSSAATG HLQASV LTQ CLKVLVKLAE NTSCDFLPRF QCVFQVLPKC LSPETPLPSV LLAVELLSLL ADHDQLAPQL CSHSEGCLLL LLYMYIT SR PDRVALETQW LQLEQEVVWL LAKLGVQSPL PPVTGSNCQC NVEVVRALTV MLHRQWLTVR RAGGPPRTDQ QRRTVRCL R DTVLLLHGLS QKDKLFMMHC VEVLHQFDQV MPGVSMLIRG LPDVTDCEEA ALDDLCAAET DVEDPEVECG UniProtKB: ATR-interacting protein |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Buffer | pH: 7.4 |
|---|---|
| Grid | Model: Quantifoil R1.2/1.3 / Material: GOLD / Mesh: 300 |
| Vitrification | Cryogen name: ETHANE |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Image recording | Film or detector model: GATAN K2 SUMMIT (4k x 4k) / Detector mode: SUPER-RESOLUTION / Digitization - Frames/image: 1-32 / Average electron dose: 50.0 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
 Movie
Movie Controller
Controller

























 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)






















