+ Open data
Open data
- Basic information
Basic information
| Entry | Database: EMDB / ID: EMD-6266 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | CryoEM structure of a type VI secretion system | |||||||||
 Map data Map data | Contracted T6SS sheath | |||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | T6SS / Francisella tularensis subsp. novicida | |||||||||
| Function / homology |  Function and homology information Function and homology information | |||||||||
| Biological species |  Francisella novicida U112 (bacteria) Francisella novicida U112 (bacteria) | |||||||||
| Method | helical reconstruction / cryo EM / Resolution: 3.7 Å | |||||||||
 Authors Authors | Clemens DL / Ge P / Lee BY / Horwitz MA / Zhou ZH | |||||||||
 Citation Citation |  Journal: Cell / Year: 2015 Journal: Cell / Year: 2015Title: Atomic structure of T6SS reveals interlaced array essential to function. Authors: Daniel L Clemens / Peng Ge / Bai-Yu Lee / Marcus A Horwitz / Z Hong Zhou /  Abstract: Type VI secretion systems (T6SSs) are newly identified contractile nanomachines that translocate effector proteins across bacterial membranes. The Francisella pathogenicity island, required for ...Type VI secretion systems (T6SSs) are newly identified contractile nanomachines that translocate effector proteins across bacterial membranes. The Francisella pathogenicity island, required for bacterial phagosome escape, intracellular replication, and virulence, was presumed to encode a T6SS-like apparatus. Here, we experimentally confirm the identity of this T6SS and, by cryo electron microscopy (cryoEM), show the structure of its post-contraction sheath at 3.7 Å resolution. We demonstrate the assembly of this T6SS by IglA/IglB and secretion of its putative effector proteins in response to environmental stimuli. The sheath has a quaternary structure with handedness opposite that of contracted sheath of T4 phage tail and is organized in an interlaced two-dimensional array by means of β sheet augmentation. By structure-based mutagenesis, we show that this interlacing is essential to secretion, phagosomal escape, and intracellular replication. Our atomic model of the T6SS will facilitate design of drugs targeting this highly prevalent secretion apparatus. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Movie |
 Movie viewer Movie viewer |
|---|---|
| Structure viewer | EM map:  SurfView SurfView Molmil Molmil Jmol/JSmol Jmol/JSmol |
| Supplemental images |
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_6266.map.gz emd_6266.map.gz | 115.3 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-6266-v30.xml emd-6266-v30.xml emd-6266.xml emd-6266.xml | 9.6 KB 9.6 KB | Display Display |  EMDB header EMDB header |
| Images |  400_6266.gif 400_6266.gif 80_6266.gif 80_6266.gif | 114.2 KB 5.7 KB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-6266 http://ftp.pdbj.org/pub/emdb/structures/EMD-6266 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-6266 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-6266 | HTTPS FTP |
-Validation report
| Summary document |  emd_6266_validation.pdf.gz emd_6266_validation.pdf.gz | 506.9 KB | Display |  EMDB validaton report EMDB validaton report |
|---|---|---|---|---|
| Full document |  emd_6266_full_validation.pdf.gz emd_6266_full_validation.pdf.gz | 506.5 KB | Display | |
| Data in XML |  emd_6266_validation.xml.gz emd_6266_validation.xml.gz | 5.9 KB | Display | |
| Arichive directory |  https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-6266 https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-6266 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-6266 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-6266 | HTTPS FTP |
-Related structure data
| Related structure data |  3j9oMC M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|
- Map
Map
| File |  Download / File: emd_6266.map.gz / Format: CCP4 / Size: 122.1 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_6266.map.gz / Format: CCP4 / Size: 122.1 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Contracted T6SS sheath | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 1 Å | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
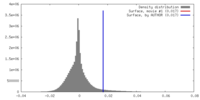
| Density |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
CCP4 map header:
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
-Supplemental data
- Sample components
Sample components
-Entire : Contracted T6SS sheath
| Entire | Name: Contracted T6SS sheath |
|---|---|
| Components |
|
-Supramolecule #1000: Contracted T6SS sheath
| Supramolecule | Name: Contracted T6SS sheath / type: sample / ID: 1000 / Oligomeric state: Helix of IglA/IglB dimers / Number unique components: 2 |
|---|
-Macromolecule #1: IglA
| Macromolecule | Name: IglA / type: protein_or_peptide / ID: 1 / Name.synonym: T6SS sheath / Recombinant expression: No / Database: NCBI |
|---|---|
| Source (natural) | Organism:  Francisella novicida U112 (bacteria) Francisella novicida U112 (bacteria) |
| Sequence | UniProtKB: Intracellular growth locus protein A |
-Macromolecule #2: IglB
| Macromolecule | Name: IglB / type: protein_or_peptide / ID: 2 / Recombinant expression: No / Database: NCBI |
|---|---|
| Source (natural) | Organism:  Francisella novicida U112 (bacteria) Francisella novicida U112 (bacteria) |
| Sequence | UniProtKB: Intracellular growth locus protein B |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | helical reconstruction |
| Aggregation state | helical array |
- Sample preparation
Sample preparation
| Buffer | pH: 7.5 / Details: 20 mM Tris, 0.9% NaCl |
|---|---|
| Grid | Details: 200 mesh Quantifoil 1.2/1.3 |
| Vitrification | Cryogen name: ETHANE / Chamber humidity: 100 % / Chamber temperature: 90 K / Instrument: FEI VITROBOT MARK IV |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Temperature | Average: 80 K |
| Alignment procedure | Legacy - Astigmatism: Software |
| Details | K2 Summit in Counting mode |
| Date | Mar 1, 2014 |
| Image recording | Category: CCD / Film or detector model: GATAN K2 (4k x 4k) / Number real images: 1644 / Average electron dose: 25 e/Å2 / Details: 480,000 asymmetric units |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD / Cs: 2.7 mm / Nominal defocus max: 3.5 µm / Nominal defocus min: 1.5 µm / Nominal magnification: 29000 |
| Sample stage | Specimen holder model: FEI TITAN KRIOS AUTOGRID HOLDER |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
- Image processing
Image processing
| Details | Relion-based IHRSR |
|---|---|
| Final reconstruction | Applied symmetry - Helical parameters - Δz: 20.8 Å Applied symmetry - Helical parameters - Δ&Phi: 33.4 ° Applied symmetry - Helical parameters - Axial symmetry: C6 (6 fold cyclic) Algorithm: OTHER / Resolution.type: BY AUTHOR / Resolution: 3.7 Å / Resolution method: OTHER / Software - Name: Relion, IHRSR |
| CTF correction | Details: each particle |
 Movie
Movie Controller
Controller







 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)