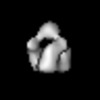[English] 日本語
 Yorodumi
Yorodumi- EMDB-5939: Electron cryo-microscopy of the partially glycosylated gp80 form ... -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: EMDB / ID: EMD-5939 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | Electron cryo-microscopy of the partially glycosylated gp80 form of the wild type furin precursor Env of Moloney murine leukemia virus | |||||||||
 Map data Map data | Reconstruction of the partially glycosylated form (gp80) of the wild type furin precursor Env of Moloney murine leukemia virus | |||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | Furin precursor / Moloney murine leukemia virus / Env maturation / gp80 | |||||||||
| Biological species |  Moloney murine leukemia virus Moloney murine leukemia virus | |||||||||
| Method | single particle reconstruction / negative staining / Resolution: 26.0 Å | |||||||||
 Authors Authors | Sjoberg M / Wu SR / Loving R / Rantalainen K / Lindqvist B / Garoff H | |||||||||
 Citation Citation |  Journal: Proc Natl Acad Sci U S A / Year: 2014 Journal: Proc Natl Acad Sci U S A / Year: 2014Title: Furin cleavage of the Moloney murine leukemia virus Env precursor reorganizes the spike structure. Authors: Mathilda Sjöberg / Shang-Rung Wu / Robin Löving / Kimmo Rantalainen / Birgitta Lindqvist / Henrik Garoff /  Abstract: The trimeric Moloney murine leukemia virus Env protein matures by two proteolytic cleavages. First, furin cleaves the Env precursor into the surface (SU) and transmembrane (TM) subunits in the cell ...The trimeric Moloney murine leukemia virus Env protein matures by two proteolytic cleavages. First, furin cleaves the Env precursor into the surface (SU) and transmembrane (TM) subunits in the cell and then the viral protease cleaves the R-peptide from TM in new virus. Here we analyzed the structure of the furin precursor, by cryoelectron microscopy. We transfected 293T cells with a furin cleavage site provirus mutant, R466G/K468G, and produced the virus in the presence of amprenavir to also inhibit the R-peptide cleavage. Although Env incorporation into particles was inhibited, enough precursor could be isolated and analyzed by cryoelectron microscopy to yield a 3D structure at 22 Å resolution. This showed an open cage-like structure like that of the R-peptide precursor and the mature Env described before. However, the middle protrusion of the protomeric unit, so prominently pointing out from the side of the more mature forms of the Env, was absent. Instead, there was extra density in the top protrusion. This suggested that the C-terminal SU domain was associated alongside the receptor binding N-terminal SU domain in the furin precursor. This was supported by mapping with a SU C-terminal domain-specific antigen binding fragment. We concluded that furin cleavage not only separates the subunits and liberates the fusion peptide at the end of TM but also allows the C-terminal domain to relocate into a peripheral position. This conformational change might explain how the C-terminal domain of SU gains the potential to undergo disulfide isomerization, an event that facilitates membrane fusion. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Movie |
 Movie viewer Movie viewer |
|---|---|
| Structure viewer | EM map:  SurfView SurfView Molmil Molmil Jmol/JSmol Jmol/JSmol |
| Supplemental images |
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_5939.map.gz emd_5939.map.gz | 345.2 KB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-5939-v30.xml emd-5939-v30.xml emd-5939.xml emd-5939.xml | 10.4 KB 10.4 KB | Display Display |  EMDB header EMDB header |
| Images |  400_5939.gif 400_5939.gif 80_5939.gif 80_5939.gif | 24.2 KB 2.7 KB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-5939 http://ftp.pdbj.org/pub/emdb/structures/EMD-5939 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-5939 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-5939 | HTTPS FTP |
-Validation report
| Summary document |  emd_5939_validation.pdf.gz emd_5939_validation.pdf.gz | 78.8 KB | Display |  EMDB validaton report EMDB validaton report |
|---|---|---|---|---|
| Full document |  emd_5939_full_validation.pdf.gz emd_5939_full_validation.pdf.gz | 77.9 KB | Display | |
| Data in XML |  emd_5939_validation.xml.gz emd_5939_validation.xml.gz | 495 B | Display | |
| Arichive directory |  https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-5939 https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-5939 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-5939 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-5939 | HTTPS FTP |
-Related structure data
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|
- Map
Map
| File |  Download / File: emd_5939.map.gz / Format: CCP4 / Size: 422.9 KB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_5939.map.gz / Format: CCP4 / Size: 422.9 KB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Reconstruction of the partially glycosylated form (gp80) of the wild type furin precursor Env of Moloney murine leukemia virus | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 3.5 Å | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Density |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
CCP4 map header:
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
-Supplemental data
- Sample components
Sample components
-Entire : Partially glycosylated form (gp80) of wild type furin precursor E...
| Entire | Name: Partially glycosylated form (gp80) of wild type furin precursor Env of Moloney murine leukemia virus |
|---|---|
| Components |
|
-Supramolecule #1000: Partially glycosylated form (gp80) of wild type furin precursor E...
| Supramolecule | Name: Partially glycosylated form (gp80) of wild type furin precursor Env of Moloney murine leukemia virus type: sample / ID: 1000 Details: Affinity purified, gradient separated protein in 0.05% Triton X-100 Oligomeric state: trimer / Number unique components: 1 |
|---|---|
| Molecular weight | Experimental: 500 KDa / Theoretical: 270 KDa / Method: Estimated from Blue native PAGE |
-Macromolecule #1: gp80
| Macromolecule | Name: gp80 / type: protein_or_peptide / ID: 1 / Name.synonym: Partially glycosylated furin precursor / Number of copies: 3 / Oligomeric state: Trimer / Recombinant expression: No / Database: NCBI |
|---|---|
| Source (natural) | Organism:  Moloney murine leukemia virus Moloney murine leukemia virus |
| Molecular weight | Experimental: 500 KDa / Theoretical: 270 KDa |
-Experimental details
-Structure determination
| Method | negative staining |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Concentration | 0.1 mg/mL |
|---|---|
| Buffer | pH: 7.4 / Details: 50 mM HEPES, 100 mM NaCl, 1.8 mM CaCl2, pH 7.4 |
| Staining | Type: NEGATIVE Details: The specimen was stained with 2% uranyl acetate (UA). |
| Grid | Details: 400 mesh holey carbon grid, glow discharged |
| Vitrification | Cryogen name: NONE / Instrument: OTHER |
- Electron microscopy
Electron microscopy
| Microscope | JEOL 2100F |
|---|---|
| Alignment procedure | Legacy - Astigmatism: Objective lens astigmatism was corrected using online FFT. |
| Date | Jan 17, 2014 |
| Image recording | Category: CCD / Film or detector model: TVIPS TEMCAM-F416 (4k x 4k) / Digitization - Sampling interval: 3.5 µm / Number real images: 30 / Average electron dose: 9 e/Å2 / Bits/pixel: 14 |
| Electron beam | Acceleration voltage: 200 kV / Electron source: TUNGSTEN HAIRPIN |
| Electron optics | Calibrated magnification: 43200 / Illumination mode: SPOT SCAN / Imaging mode: BRIGHT FIELD / Cs: 2.0 mm / Nominal defocus max: 3.072 µm / Nominal defocus min: 2.048 µm / Nominal magnification: 43200 |
| Sample stage | Specimen holder model: OTHER |
 Movie
Movie Controller
Controller


 UCSF Chimera
UCSF Chimera








 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)