+ Open data
Open data
- Basic information
Basic information
| Entry |  | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Title | Structure of the undecorated pointed end of F-actin | ||||||||||||
 Map data Map data | Sharpened cryo-EM density map of the undecorated pointed end of actin filaments. | ||||||||||||
 Sample Sample |
| ||||||||||||
 Keywords Keywords | actin / filament / pointed end / STRUCTURAL PROTEIN | ||||||||||||
| Function / homology |  Function and homology information Function and homology informationcytoskeletal motor activator activity / myosin heavy chain binding / tropomyosin binding / actin filament bundle / troponin I binding / filamentous actin / mesenchyme migration / actin filament bundle assembly / skeletal muscle myofibril / striated muscle thin filament ...cytoskeletal motor activator activity / myosin heavy chain binding / tropomyosin binding / actin filament bundle / troponin I binding / filamentous actin / mesenchyme migration / actin filament bundle assembly / skeletal muscle myofibril / striated muscle thin filament / skeletal muscle thin filament assembly / actin monomer binding / skeletal muscle fiber development / stress fiber / titin binding / actin filament polymerization / actin filament / filopodium / Hydrolases; Acting on acid anhydrides; Acting on acid anhydrides to facilitate cellular and subcellular movement / calcium-dependent protein binding / lamellipodium / cell body / hydrolase activity / protein domain specific binding / calcium ion binding / positive regulation of gene expression / magnesium ion binding / ATP binding / identical protein binding / cytoplasm Similarity search - Function | ||||||||||||
| Biological species |  | ||||||||||||
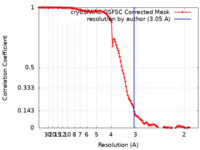
| Method | single particle reconstruction / cryo EM / Resolution: 3.05 Å | ||||||||||||
 Authors Authors | Boiero Sanders M / Oosterheert W / Hofnagel O / Bieling P / Raunser S | ||||||||||||
| Funding support |  Germany, European Union, 3 items Germany, European Union, 3 items
| ||||||||||||
 Citation Citation |  Journal: Nat Commun / Year: 2024 Journal: Nat Commun / Year: 2024Title: Phalloidin and DNase I-bound F-actin pointed end structures reveal principles of filament stabilization and disassembly. Authors: Micaela Boiero Sanders / Wout Oosterheert / Oliver Hofnagel / Peter Bieling / Stefan Raunser /  Abstract: Actin filament turnover involves subunits binding to and dissociating from the filament ends, with the pointed end being the primary site of filament disassembly. Several molecules modulate filament ...Actin filament turnover involves subunits binding to and dissociating from the filament ends, with the pointed end being the primary site of filament disassembly. Several molecules modulate filament turnover, but the underlying mechanisms remain incompletely understood. Here, we present three cryo-EM structures of the F-actin pointed end in the presence and absence of phalloidin or DNase I. The two terminal subunits at the undecorated pointed end adopt a twisted conformation. Phalloidin can still bind and bridge these subunits, inducing a conformational shift to a flattened, F-actin-like state. This explains how phalloidin prevents depolymerization at the pointed end. Interestingly, two DNase I molecules simultaneously bind to the phalloidin-stabilized pointed end. In the absence of phalloidin, DNase I binding would disrupt the terminal actin subunit packing, resulting in filament disassembly. Our findings uncover molecular principles of pointed end regulation and provide structural insights into the kinetic asymmetry between the actin filament ends. | ||||||||||||
| History |
|
- Structure visualization
Structure visualization
| Supplemental images |
|---|
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_50507.map.gz emd_50507.map.gz | 168 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-50507-v30.xml emd-50507-v30.xml emd-50507.xml emd-50507.xml | 23.2 KB 23.2 KB | Display Display |  EMDB header EMDB header |
| FSC (resolution estimation) |  emd_50507_fsc.xml emd_50507_fsc.xml | 11.9 KB | Display |  FSC data file FSC data file |
| Images |  emd_50507.png emd_50507.png | 131.3 KB | ||
| Masks |  emd_50507_msk_1.map emd_50507_msk_1.map | 178 MB |  Mask map Mask map | |
| Filedesc metadata |  emd-50507.cif.gz emd-50507.cif.gz | 6.7 KB | ||
| Others |  emd_50507_additional_1.map.gz emd_50507_additional_1.map.gz emd_50507_half_map_1.map.gz emd_50507_half_map_1.map.gz emd_50507_half_map_2.map.gz emd_50507_half_map_2.map.gz | 89.2 MB 165.3 MB 165.3 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-50507 http://ftp.pdbj.org/pub/emdb/structures/EMD-50507 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-50507 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-50507 | HTTPS FTP |
-Validation report
| Summary document |  emd_50507_validation.pdf.gz emd_50507_validation.pdf.gz | 1.1 MB | Display |  EMDB validaton report EMDB validaton report |
|---|---|---|---|---|
| Full document |  emd_50507_full_validation.pdf.gz emd_50507_full_validation.pdf.gz | 1.1 MB | Display | |
| Data in XML |  emd_50507_validation.xml.gz emd_50507_validation.xml.gz | 20.5 KB | Display | |
| Data in CIF |  emd_50507_validation.cif.gz emd_50507_validation.cif.gz | 26.6 KB | Display | |
| Arichive directory |  https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-50507 https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-50507 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-50507 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-50507 | HTTPS FTP |
-Related structure data
| Related structure data |  9fjoM C: citing same article ( M: atomic model generated by this map |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_50507.map.gz / Format: CCP4 / Size: 178 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_50507.map.gz / Format: CCP4 / Size: 178 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Sharpened cryo-EM density map of the undecorated pointed end of actin filaments. | ||||||||||||||||||||||||||||||||||||
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 0.9 Å | ||||||||||||||||||||||||||||||||||||
| Density |
| ||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
|
-Supplemental data
-Mask #1
| File |  emd_50507_msk_1.map emd_50507_msk_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
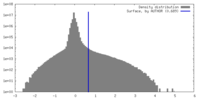
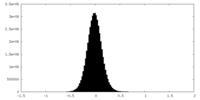
| Density Histograms |
-Additional map: Unsharpened cryo-EM density map of the undecorated pointed...
| File | emd_50507_additional_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Unsharpened cryo-EM density map of the undecorated pointed end of actin filaments. | ||||||||||||
| Projections & Slices |
| ||||||||||||
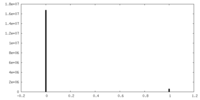
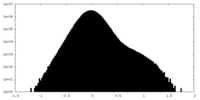
| Density Histograms |
-Half map: Unfiltered half map 1 of the undecorated pointed...
| File | emd_50507_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Unfiltered half map 1 of the undecorated pointed end of actin filaments. | ||||||||||||
| Projections & Slices |
| ||||||||||||
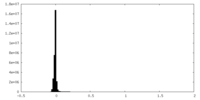
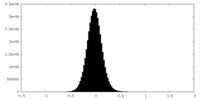
| Density Histograms |
-Half map: Unfiltered half map 2 of the undecorated pointed...
| File | emd_50507_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Unfiltered half map 2 of the undecorated pointed end of actin filaments. | ||||||||||||
| Projections & Slices |
| ||||||||||||
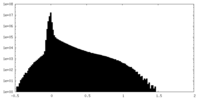
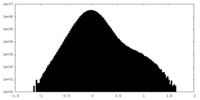
| Density Histograms |
- Sample components
Sample components
-Entire : Complex of actin subunits that form the pointed end of actin fila...
| Entire | Name: Complex of actin subunits that form the pointed end of actin filaments. |
|---|---|
| Components |
|
-Supramolecule #1: Complex of actin subunits that form the pointed end of actin fila...
| Supramolecule | Name: Complex of actin subunits that form the pointed end of actin filaments. type: complex / ID: 1 / Parent: 0 / Macromolecule list: #1 Details: Alpha actin was purified from skeletal muscle. Actin filaments were polymerized in the presence of the formin INF2, which was purified separetely and added during polymerization prior to ...Details: Alpha actin was purified from skeletal muscle. Actin filaments were polymerized in the presence of the formin INF2, which was purified separetely and added during polymerization prior to cryo-EM grid preparation. |
|---|---|
| Source (natural) | Organism:  |
-Macromolecule #1: Actin, alpha skeletal muscle
| Macromolecule | Name: Actin, alpha skeletal muscle / type: protein_or_peptide / ID: 1 Details: Rabbit skeletal alpha actin purified from frozen rabbit muscle acetone powder. Number of copies: 4 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  |
| Molecular weight | Theoretical: 41.875633 KDa |
| Sequence | String: DEDETTALVC DNGSGLVKAG FAGDDAPRAV FPSIVGRPRH QGVMVGMGQK DSYVGDEAQS KRGILTLKYP IE(HIC)GII TNW DDMEKIWHHT FYNELRVAPE EHPTLLTEAP LNPKANREKM TQIMFETFNV PAMYVAIQAV LSLYASGRTT GIVLDSG DG VTHNVPIYEG ...String: DEDETTALVC DNGSGLVKAG FAGDDAPRAV FPSIVGRPRH QGVMVGMGQK DSYVGDEAQS KRGILTLKYP IE(HIC)GII TNW DDMEKIWHHT FYNELRVAPE EHPTLLTEAP LNPKANREKM TQIMFETFNV PAMYVAIQAV LSLYASGRTT GIVLDSG DG VTHNVPIYEG YALPHAIMRL DLAGRDLTDY LMKILTERGY SFVTTAEREI VRDIKEKLCY VALDFENEMA TAASSSSL E KSYELPDGQV ITIGNERFRC PETLFQPSFI GMESAGIHET TYNSIMKCDI DIRKDLYANN VMSGGTTMYP GIADRMQKE ITALAPSTMK IKIIAPPERK YSVWIGGSIL ASLSTFQQMW ITKQEYDEAG PSIVHRKCF UniProtKB: Actin, alpha skeletal muscle |
-Macromolecule #2: ADENOSINE-5'-DIPHOSPHATE
| Macromolecule | Name: ADENOSINE-5'-DIPHOSPHATE / type: ligand / ID: 2 / Number of copies: 4 / Formula: ADP |
|---|---|
| Molecular weight | Theoretical: 427.201 Da |
| Chemical component information |  ChemComp-ADP: |
-Macromolecule #3: MAGNESIUM ION
| Macromolecule | Name: MAGNESIUM ION / type: ligand / ID: 3 / Number of copies: 4 / Formula: MG |
|---|---|
| Molecular weight | Theoretical: 24.305 Da |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Buffer | pH: 7.1 Component:
Details: 12 mM HEPES pH 7.1, 100 mM KCl, 2.1 mM MgCl2, 1 mM EGTA, 1 mM TCEP, 0.2 mM ATP | |||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Grid | Model: Quantifoil R2/1 / Material: GOLD / Mesh: 200 / Support film - Material: CELLULOSE ACETATE / Support film - topology: HOLEY / Pretreatment - Type: GLOW DISCHARGE / Pretreatment - Time: 90 sec. | |||||||||||||||||||||
| Vitrification | Cryogen name: ETHANE-PROPANE / Chamber humidity: 100 % / Chamber temperature: 286 K / Instrument: FEI VITROBOT MARK IV / Details: 3 seconds, force 0.. |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Specialist optics | Spherical aberration corrector: 300 kV Titan Krios G3 microscope (Thermo Fisher Scientific). Energy filter - Name: GIF Bioquantum / Energy filter - Slit width: 15 eV / Details: Gatan energy filter |
| Details | 300 kV Titan Krios G3 microscope (Thermo Fisher Scientific). |
| Image recording | Film or detector model: GATAN K3 BIOQUANTUM (6k x 4k) / Number grids imaged: 1 / Number real images: 20305 / Average electron dose: 60.6 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | C2 aperture diameter: 50.0 µm / Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD / Cs: 2.7 mm / Nominal defocus max: 2.9 µm / Nominal defocus min: 1.3 µm / Nominal magnification: 105000 |
| Sample stage | Specimen holder model: FEI TITAN KRIOS AUTOGRID HOLDER / Cooling holder cryogen: NITROGEN |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
 Movie
Movie Controller
Controller












 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)