+ データを開く
データを開く
- 基本情報
基本情報
| 登録情報 | データベース: EMDB / ID: EMD-4391 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| タイトル | Structure of P-glycoprotein(ABCB1) in the post-hydrolytic state | |||||||||
 マップデータ マップデータ | Shows the additional density compared to the core domains as discussed in the manuscript. Corresponds to a volume enclosed of 160x10^3 Angstrom cubed. | |||||||||
 試料 試料 |
| |||||||||
 キーワード キーワード | P-glycoprotein / ABCB1 / ATP-binding cassette / transporter / membrane protein / protein structure | |||||||||
| 機能・相同性 |  機能・相同性情報 機能・相同性情報Atorvastatin ADME / Prednisone ADME / hormone transport / cellular response to nonylphenol / cellular response to borneol / response to codeine / response to cyclosporin A / cellular response to mycotoxin / daunorubicin transport / positive regulation of response to drug ...Atorvastatin ADME / Prednisone ADME / hormone transport / cellular response to nonylphenol / cellular response to borneol / response to codeine / response to cyclosporin A / cellular response to mycotoxin / daunorubicin transport / positive regulation of response to drug / terpenoid transport / ceramide floppase activity / negative regulation of sensory perception of pain / positive regulation of establishment of Sertoli cell barrier / regulation of intestinal absorption / cellular response to external biotic stimulus / response to quercetin / response to antineoplastic agent / ceramide translocation / floppase activity / ABC-family proteins mediated transport / establishment of blood-retinal barrier / protein localization to bicellular tight junction / phosphatidylethanolamine flippase activity / phosphatidylcholine floppase activity / xenobiotic transport across blood-brain barrier / response to thyroxine / establishment of blood-brain barrier / intercellular canaliculus / xenobiotic detoxification by transmembrane export across the plasma membrane / export across plasma membrane / P-type phospholipid transporter / cellular response to L-glutamate / ABC-type xenobiotic transporter / response to vitamin A / response to vitamin D / response to glucagon / response to alcohol / response to glycoside / intestinal absorption / ABC-type xenobiotic transporter activity / cellular response to antibiotic / phospholipid translocation / cellular hyperosmotic salinity response / maintenance of blood-brain barrier / cellular response to alkaloid / efflux transmembrane transporter activity / ATPase-coupled transmembrane transporter activity / transmembrane transporter activity / xenobiotic transmembrane transporter activity / cellular response to dexamethasone stimulus / response to cadmium ion / lactation / response to progesterone / placenta development / cellular response to estradiol stimulus / brush border membrane / female pregnancy / circadian rhythm / cellular response to tumor necrosis factor / cellular response to lipopolysaccharide / response to hypoxia / apical plasma membrane / response to xenobiotic stimulus / ATP hydrolysis activity / ATP binding / plasma membrane / cytoplasm 類似検索 - 分子機能 | |||||||||
| 生物種 |  | |||||||||
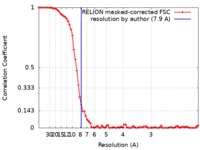
| 手法 | 単粒子再構成法 / クライオ電子顕微鏡法 / 解像度: 7.9 Å | |||||||||
 データ登録者 データ登録者 | Ford RC / Thonghin N | |||||||||
 引用 引用 |  ジャーナル: BMC Struct Biol / 年: 2018 ジャーナル: BMC Struct Biol / 年: 2018タイトル: Novel features in the structure of P-glycoprotein (ABCB1) in the post-hydrolytic state as determined at 7.9 Å resolution. 著者: Nopnithi Thonghin / Richard F Collins / Alessandro Barbieri / Talha Shafi / Alistair Siebert / Robert C Ford /  要旨: BACKGROUND: P-glycoprotein (ABCB1) is an ATP-binding cassette transporter that plays an important role in the clearance of drugs and xenobiotics and is associated with multi-drug resistance in cancer. ...BACKGROUND: P-glycoprotein (ABCB1) is an ATP-binding cassette transporter that plays an important role in the clearance of drugs and xenobiotics and is associated with multi-drug resistance in cancer. Although several P-glycoprotein structures are available, these are either at low resolution, or represent mutated and/or quiescent states of the protein. RESULTS: In the post-hydrolytic state the structure of the wild-type protein has been resolved at about 8 Å resolution. The cytosolic nucleotide-binding domains (NBDs) are separated but ADP ...RESULTS: In the post-hydrolytic state the structure of the wild-type protein has been resolved at about 8 Å resolution. The cytosolic nucleotide-binding domains (NBDs) are separated but ADP remains bound, especially at the first NBD. Gaps in the transmembrane domains (TMDs) that connect to an inner hydrophilic cavity are filled by density emerging from the annular detergent micelle. The NBD-TMD linker is partly resolved, being located between the NBDs and close to the Signature regions involved in cooperative NBD dimerization. This, and the gap-filling detergent suggest steric impediment to NBD dimerization in the post-hydrolytic state. Two central regions of density lie in two predicted drug-binding sites, implying that the protein may adventitiously bind hydrophobic substances even in the post-hydrolytic state. The previously unresolved N-terminal extension was observed, and the data suggests these 30 residues interact with the headgroup region of the lipid bilayer. CONCLUSION: The structural data imply that (i) a low basal ATPase activity is ensured by steric blockers of NBD dimerization and (ii) allocrite access to the central cavity may be structurally linked ...CONCLUSION: The structural data imply that (i) a low basal ATPase activity is ensured by steric blockers of NBD dimerization and (ii) allocrite access to the central cavity may be structurally linked to NBD dimerization, giving insights into the mechanism of drug-stimulation of P-glycoprotein activity. | |||||||||
| 履歴 |
|
- 構造の表示
構造の表示
| ムービー |
 ムービービューア ムービービューア |
|---|---|
| 構造ビューア | EMマップ:  SurfView SurfView Molmil Molmil Jmol/JSmol Jmol/JSmol |
| 添付画像 |
- ダウンロードとリンク
ダウンロードとリンク
-EMDBアーカイブ
| マップデータ |  emd_4391.map.gz emd_4391.map.gz | 6.1 MB |  EMDBマップデータ形式 EMDBマップデータ形式 | |
|---|---|---|---|---|
| ヘッダ (付随情報) |  emd-4391-v30.xml emd-4391-v30.xml emd-4391.xml emd-4391.xml | 11.4 KB 11.4 KB | 表示 表示 |  EMDBヘッダ EMDBヘッダ |
| FSC (解像度算出) |  emd_4391_fsc.xml emd_4391_fsc.xml | 7.6 KB | 表示 |  FSCデータファイル FSCデータファイル |
| 画像 |  emd_4391.png emd_4391.png | 44.9 KB | ||
| Filedesc metadata |  emd-4391.cif.gz emd-4391.cif.gz | 6.1 KB | ||
| アーカイブディレクトリ |  http://ftp.pdbj.org/pub/emdb/structures/EMD-4391 http://ftp.pdbj.org/pub/emdb/structures/EMD-4391 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-4391 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-4391 | HTTPS FTP |
-検証レポート
| 文書・要旨 |  emd_4391_validation.pdf.gz emd_4391_validation.pdf.gz | 252.7 KB | 表示 |  EMDB検証レポート EMDB検証レポート |
|---|---|---|---|---|
| 文書・詳細版 |  emd_4391_full_validation.pdf.gz emd_4391_full_validation.pdf.gz | 251.9 KB | 表示 | |
| XML形式データ |  emd_4391_validation.xml.gz emd_4391_validation.xml.gz | 9.5 KB | 表示 | |
| アーカイブディレクトリ |  https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-4391 https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-4391 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-4391 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-4391 | HTTPS FTP |
-関連構造データ
- リンク
リンク
| EMDBのページ |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| 「今月の分子」の関連する項目 |
- マップ
マップ
| ファイル |  ダウンロード / ファイル: emd_4391.map.gz / 形式: CCP4 / 大きさ: 14.5 MB / タイプ: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) ダウンロード / ファイル: emd_4391.map.gz / 形式: CCP4 / 大きさ: 14.5 MB / タイプ: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 注釈 | Shows the additional density compared to the core domains as discussed in the manuscript. Corresponds to a volume enclosed of 160x10^3 Angstrom cubed. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 投影像・断面図 | 画像のコントロール
画像は Spider により作成 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| ボクセルのサイズ | X=Y=Z: 1.06 Å | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
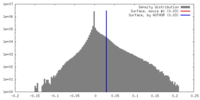
| 密度 |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 対称性 | 空間群: 1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 詳細 | EMDB XML:
CCP4マップ ヘッダ情報:
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
-添付データ
- 試料の構成要素
試料の構成要素
-全体 : mouse P-glycoprotein
| 全体 | 名称: mouse P-glycoprotein |
|---|---|
| 要素 |
|
-超分子 #1: mouse P-glycoprotein
| 超分子 | 名称: mouse P-glycoprotein / タイプ: complex / ID: 1 / 親要素: 0 / 含まれる分子: all |
|---|---|
| 由来(天然) | 生物種:  |
-分子 #1: Multidrug resistance protein 1A
| 分子 | 名称: Multidrug resistance protein 1A / タイプ: protein_or_peptide / ID: 1 / コピー数: 1 / 光学異性体: LEVO / EC番号: ec: 3.6.3.44 |
|---|---|
| 由来(天然) | 生物種:  |
| 分子量 | 理論値: 141.877875 KDa |
| 組換発現 | 生物種:  Komagataella pastoris (菌類) Komagataella pastoris (菌類) |
| 配列 | 文字列: MELEEDLKGR ADKNFSKMGK KSKKEKKEKK PAVSVLTMFR YAGWLDRLYM LVGTLAAIIH GVALPLMMLI FGDMTDSFAS VGNVSKNST NMSEADKRAM FAKLEEEMTT YAYYYTGIGA GVLIVAYIQV SFWCLAAGRQ IHKIRQKFFH AIMNQEIGWF D VHDVGELN ...文字列: MELEEDLKGR ADKNFSKMGK KSKKEKKEKK PAVSVLTMFR YAGWLDRLYM LVGTLAAIIH GVALPLMMLI FGDMTDSFAS VGNVSKNST NMSEADKRAM FAKLEEEMTT YAYYYTGIGA GVLIVAYIQV SFWCLAAGRQ IHKIRQKFFH AIMNQEIGWF D VHDVGELN TRLTDDVSKI NEGIGDKIGM FFQAMATFFG GFIIGFTRGW KLTLVILAIS PVLGLSAGIW AKILSSFTDK EL HAYAKAG AVAEEVLAAI RTVIAFGGQK KELERYNNNL EEAKRLGIKK AITANISMGA AFLLIYASYA LAFWYGTSLV ISK EYSIGQ VLTVFFSVLI GAFSVGQASP NIEAFANARG AAYEVFKIID NKPSIDSFSK SGHKPDNIQG NLEFKNIHFS YPSR KEVQI LKGLNLKVKS GQTVALVGNS GCGKSTTVQL MQRLYDPLDG MVSIDGQDIR TINVRYLREI IGVVSQEPVL FATTI AENI RYGREDVTMD EIEKAVKEAN AYDFIMKLPH QFDTLVGERG AQLSGGQKQR IAIARALVRN PKILLLDEAT SALDTE SEA VVQAALDKAR EGRTTIVIAH RLSTVRNADV IAGFDGGVIV EQGNHDELMR EKGIYFKLVM TQTAGNEIEL GNEACKS KD EIDNLDMSSK DSGSSLIRRR STRKSICGPH DQDRKLSTKE ALDEDVPPAS FWRILKLNST EWPYFVVGIF CAIINGGL Q PAFSVIFSKV VGVFTNGGPP ETQRQNSNLF SLLFLILGII SFITFFLQGF TFGKAGEILT KRLRYMVFKS MLRQDVSWF DDPKNTTGAL TTRLANDAAQ VKGATGSRLA VIFQNIANLG TGIIISLIYG WQLTLLLLAI VPIIAIAGVV EMKMLSGQAL KDKKELEGS GKIATEAIEN FRTVVSLTRE QKFETMYAQS LQIPYRNAMK KAHVFGITFS FTQAMMYFSY AACFRFGAYL V TQQLMTFE NVLLVFSAIV FGAMAVGQVS SFAPDYAKAT VSASHIIRII EKTPEIDSYS TQGLKPNMLE GNVQFSGVVF NY PTRPSIP VLQGLSLEVK KGQTLALVGS SGCGKSTVVQ LLERFYDPMA GSVFLDGKEI KQLNVQWLRA QLGIVSQEPI LFD CSIAEN IAYGDNSRVV SYEEIVRAAK EANIHQFIDS LPDKYNTRVG DKGTQLSGGQ KQRIAIARAL VRQPHILLLD EATS ALDTE SEKVVQEALD KAREGRTCIV IAHRLSTIQN ADLIVVIQNG KVKEHGTHQQ LLAQKGIYFS MVSVQAGAKR SLEHH HHHH UniProtKB: ATP-dependent translocase ABCB1 |
-実験情報
-構造解析
| 手法 | クライオ電子顕微鏡法 |
|---|---|
 解析 解析 | 単粒子再構成法 |
| 試料の集合状態 | particle |
- 試料調製
試料調製
| 濃度 | 1.1 mg/mL |
|---|---|
| 緩衝液 | pH: 8 |
| グリッド | モデル: Quantifoil R1.2/1.3 / 材質: GOLD / メッシュ: 200 / 支持フィルム - 材質: CARBON / 支持フィルム - トポロジー: HOLEY / 前処理 - タイプ: GLOW DISCHARGE / 前処理 - 時間: 120 sec. / 前処理 - 雰囲気: AIR |
| 凍結 | 凍結剤: ETHANE / チャンバー内湿度: 95 % / チャンバー内温度: 295 K / 装置: FEI VITROBOT MARK IV |
- 電子顕微鏡法
電子顕微鏡法
| 顕微鏡 | FEI TITAN KRIOS |
|---|---|
| 撮影 | フィルム・検出器のモデル: GATAN K2 SUMMIT (4k x 4k) 平均電子線量: 70.0 e/Å2 |
| 電子線 | 加速電圧: 300 kV / 電子線源:  FIELD EMISSION GUN FIELD EMISSION GUN |
| 電子光学系 | 照射モード: FLOOD BEAM / 撮影モード: BRIGHT FIELD |
| 実験機器 |  モデル: Titan Krios / 画像提供: FEI Company |
+ 画像解析
画像解析
-原子モデル構築 1
| 初期モデル | PDB ID: Chain - Chain ID: a / Chain - Residue range: 33-1271 / Chain - Source name: PDB / Chain - Initial model type: experimental model |
|---|---|
| 精密化 | プロトコル: FLEXIBLE FIT |
| 得られたモデル |  PDB-6gdi:  PDB-6q81: |
 ムービー
ムービー コントローラー
コントローラー
















 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)