+ Open data
Open data
- Basic information
Basic information
| Entry | Database: EMDB / ID: EMD-4391 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | Structure of P-glycoprotein(ABCB1) in the post-hydrolytic state | |||||||||
 Map data Map data | Shows the additional density compared to the core domains as discussed in the manuscript. Corresponds to a volume enclosed of 160x10^3 Angstrom cubed. | |||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | P-glycoprotein / ABCB1 / ATP-binding cassette / transporter / membrane protein / protein structure | |||||||||
| Function / homology |  Function and homology information Function and homology informationAtorvastatin ADME / Prednisone ADME / hormone transport / cellular response to nonylphenol / cellular response to borneol / response to codeine / response to cyclosporin A / cellular response to mycotoxin / daunorubicin transport / positive regulation of response to drug ...Atorvastatin ADME / Prednisone ADME / hormone transport / cellular response to nonylphenol / cellular response to borneol / response to codeine / response to cyclosporin A / cellular response to mycotoxin / daunorubicin transport / positive regulation of response to drug / terpenoid transport / ceramide floppase activity / negative regulation of sensory perception of pain / positive regulation of establishment of Sertoli cell barrier / regulation of intestinal absorption / cellular response to external biotic stimulus / response to quercetin / response to antineoplastic agent / floppase activity / ceramide translocation / ABC-family proteins mediated transport / establishment of blood-retinal barrier / protein localization to bicellular tight junction / phosphatidylethanolamine flippase activity / phosphatidylcholine floppase activity / xenobiotic transport across blood-brain barrier / response to thyroxine / establishment of blood-brain barrier / intercellular canaliculus / xenobiotic detoxification by transmembrane export across the plasma membrane / export across plasma membrane / P-type phospholipid transporter / cellular response to L-glutamate / response to vitamin A / ABC-type xenobiotic transporter / response to vitamin D / response to glucagon / response to alcohol / intestinal absorption / response to glycoside / ABC-type xenobiotic transporter activity / cellular response to antibiotic / phospholipid translocation / cellular hyperosmotic salinity response / maintenance of blood-brain barrier / cellular response to alkaloid / efflux transmembrane transporter activity / ATPase-coupled transmembrane transporter activity / xenobiotic transmembrane transporter activity / transmembrane transporter activity / cellular response to dexamethasone stimulus / response to cadmium ion / lactation / response to progesterone / placenta development / cellular response to estradiol stimulus / brush border membrane / female pregnancy / circadian rhythm / cellular response to tumor necrosis factor / cellular response to lipopolysaccharide / response to hypoxia / apical plasma membrane / response to xenobiotic stimulus / ATP hydrolysis activity / ATP binding / plasma membrane / cytoplasm Similarity search - Function | |||||||||
| Biological species |  | |||||||||
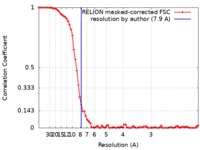
| Method | single particle reconstruction / cryo EM / Resolution: 7.9 Å | |||||||||
 Authors Authors | Ford RC / Thonghin N | |||||||||
 Citation Citation |  Journal: BMC Struct Biol / Year: 2018 Journal: BMC Struct Biol / Year: 2018Title: Novel features in the structure of P-glycoprotein (ABCB1) in the post-hydrolytic state as determined at 7.9 Å resolution. Authors: Nopnithi Thonghin / Richard F Collins / Alessandro Barbieri / Talha Shafi / Alistair Siebert / Robert C Ford /  Abstract: BACKGROUND: P-glycoprotein (ABCB1) is an ATP-binding cassette transporter that plays an important role in the clearance of drugs and xenobiotics and is associated with multi-drug resistance in cancer. ...BACKGROUND: P-glycoprotein (ABCB1) is an ATP-binding cassette transporter that plays an important role in the clearance of drugs and xenobiotics and is associated with multi-drug resistance in cancer. Although several P-glycoprotein structures are available, these are either at low resolution, or represent mutated and/or quiescent states of the protein. RESULTS: In the post-hydrolytic state the structure of the wild-type protein has been resolved at about 8 Å resolution. The cytosolic nucleotide-binding domains (NBDs) are separated but ADP ...RESULTS: In the post-hydrolytic state the structure of the wild-type protein has been resolved at about 8 Å resolution. The cytosolic nucleotide-binding domains (NBDs) are separated but ADP remains bound, especially at the first NBD. Gaps in the transmembrane domains (TMDs) that connect to an inner hydrophilic cavity are filled by density emerging from the annular detergent micelle. The NBD-TMD linker is partly resolved, being located between the NBDs and close to the Signature regions involved in cooperative NBD dimerization. This, and the gap-filling detergent suggest steric impediment to NBD dimerization in the post-hydrolytic state. Two central regions of density lie in two predicted drug-binding sites, implying that the protein may adventitiously bind hydrophobic substances even in the post-hydrolytic state. The previously unresolved N-terminal extension was observed, and the data suggests these 30 residues interact with the headgroup region of the lipid bilayer. CONCLUSION: The structural data imply that (i) a low basal ATPase activity is ensured by steric blockers of NBD dimerization and (ii) allocrite access to the central cavity may be structurally linked ...CONCLUSION: The structural data imply that (i) a low basal ATPase activity is ensured by steric blockers of NBD dimerization and (ii) allocrite access to the central cavity may be structurally linked to NBD dimerization, giving insights into the mechanism of drug-stimulation of P-glycoprotein activity. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Movie |
 Movie viewer Movie viewer |
|---|---|
| Structure viewer | EM map:  SurfView SurfView Molmil Molmil Jmol/JSmol Jmol/JSmol |
| Supplemental images |
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_4391.map.gz emd_4391.map.gz | 6.1 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-4391-v30.xml emd-4391-v30.xml emd-4391.xml emd-4391.xml | 11.4 KB 11.4 KB | Display Display |  EMDB header EMDB header |
| FSC (resolution estimation) |  emd_4391_fsc.xml emd_4391_fsc.xml | 7.6 KB | Display |  FSC data file FSC data file |
| Images |  emd_4391.png emd_4391.png | 44.9 KB | ||
| Filedesc metadata |  emd-4391.cif.gz emd-4391.cif.gz | 6.1 KB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-4391 http://ftp.pdbj.org/pub/emdb/structures/EMD-4391 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-4391 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-4391 | HTTPS FTP |
-Related structure data
| Related structure data |  6gdiMC  6q81MC M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_4391.map.gz / Format: CCP4 / Size: 14.5 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_4391.map.gz / Format: CCP4 / Size: 14.5 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Shows the additional density compared to the core domains as discussed in the manuscript. Corresponds to a volume enclosed of 160x10^3 Angstrom cubed. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 1.06 Å | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Density |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
CCP4 map header:
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
-Supplemental data
- Sample components
Sample components
-Entire : mouse P-glycoprotein
| Entire | Name: mouse P-glycoprotein |
|---|---|
| Components |
|
-Supramolecule #1: mouse P-glycoprotein
| Supramolecule | Name: mouse P-glycoprotein / type: complex / ID: 1 / Parent: 0 / Macromolecule list: all |
|---|---|
| Source (natural) | Organism:  |
-Macromolecule #1: Multidrug resistance protein 1A
| Macromolecule | Name: Multidrug resistance protein 1A / type: protein_or_peptide / ID: 1 / Number of copies: 1 / Enantiomer: LEVO / EC number: ec: 3.6.3.44 |
|---|---|
| Source (natural) | Organism:  |
| Molecular weight | Theoretical: 141.877875 KDa |
| Recombinant expression | Organism:  Komagataella pastoris (fungus) Komagataella pastoris (fungus) |
| Sequence | String: MELEEDLKGR ADKNFSKMGK KSKKEKKEKK PAVSVLTMFR YAGWLDRLYM LVGTLAAIIH GVALPLMMLI FGDMTDSFAS VGNVSKNST NMSEADKRAM FAKLEEEMTT YAYYYTGIGA GVLIVAYIQV SFWCLAAGRQ IHKIRQKFFH AIMNQEIGWF D VHDVGELN ...String: MELEEDLKGR ADKNFSKMGK KSKKEKKEKK PAVSVLTMFR YAGWLDRLYM LVGTLAAIIH GVALPLMMLI FGDMTDSFAS VGNVSKNST NMSEADKRAM FAKLEEEMTT YAYYYTGIGA GVLIVAYIQV SFWCLAAGRQ IHKIRQKFFH AIMNQEIGWF D VHDVGELN TRLTDDVSKI NEGIGDKIGM FFQAMATFFG GFIIGFTRGW KLTLVILAIS PVLGLSAGIW AKILSSFTDK EL HAYAKAG AVAEEVLAAI RTVIAFGGQK KELERYNNNL EEAKRLGIKK AITANISMGA AFLLIYASYA LAFWYGTSLV ISK EYSIGQ VLTVFFSVLI GAFSVGQASP NIEAFANARG AAYEVFKIID NKPSIDSFSK SGHKPDNIQG NLEFKNIHFS YPSR KEVQI LKGLNLKVKS GQTVALVGNS GCGKSTTVQL MQRLYDPLDG MVSIDGQDIR TINVRYLREI IGVVSQEPVL FATTI AENI RYGREDVTMD EIEKAVKEAN AYDFIMKLPH QFDTLVGERG AQLSGGQKQR IAIARALVRN PKILLLDEAT SALDTE SEA VVQAALDKAR EGRTTIVIAH RLSTVRNADV IAGFDGGVIV EQGNHDELMR EKGIYFKLVM TQTAGNEIEL GNEACKS KD EIDNLDMSSK DSGSSLIRRR STRKSICGPH DQDRKLSTKE ALDEDVPPAS FWRILKLNST EWPYFVVGIF CAIINGGL Q PAFSVIFSKV VGVFTNGGPP ETQRQNSNLF SLLFLILGII SFITFFLQGF TFGKAGEILT KRLRYMVFKS MLRQDVSWF DDPKNTTGAL TTRLANDAAQ VKGATGSRLA VIFQNIANLG TGIIISLIYG WQLTLLLLAI VPIIAIAGVV EMKMLSGQAL KDKKELEGS GKIATEAIEN FRTVVSLTRE QKFETMYAQS LQIPYRNAMK KAHVFGITFS FTQAMMYFSY AACFRFGAYL V TQQLMTFE NVLLVFSAIV FGAMAVGQVS SFAPDYAKAT VSASHIIRII EKTPEIDSYS TQGLKPNMLE GNVQFSGVVF NY PTRPSIP VLQGLSLEVK KGQTLALVGS SGCGKSTVVQ LLERFYDPMA GSVFLDGKEI KQLNVQWLRA QLGIVSQEPI LFD CSIAEN IAYGDNSRVV SYEEIVRAAK EANIHQFIDS LPDKYNTRVG DKGTQLSGGQ KQRIAIARAL VRQPHILLLD EATS ALDTE SEKVVQEALD KAREGRTCIV IAHRLSTIQN ADLIVVIQNG KVKEHGTHQQ LLAQKGIYFS MVSVQAGAKR SLEHH HHHH UniProtKB: ATP-dependent translocase ABCB1 |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Concentration | 1.1 mg/mL |
|---|---|
| Buffer | pH: 8 |
| Grid | Model: Quantifoil R1.2/1.3 / Material: GOLD / Mesh: 200 / Support film - Material: CARBON / Support film - topology: HOLEY / Pretreatment - Type: GLOW DISCHARGE / Pretreatment - Time: 120 sec. / Pretreatment - Atmosphere: AIR |
| Vitrification | Cryogen name: ETHANE / Chamber humidity: 95 % / Chamber temperature: 295 K / Instrument: FEI VITROBOT MARK IV |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Image recording | Film or detector model: GATAN K2 SUMMIT (4k x 4k) / Average electron dose: 70.0 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
+ Image processing
Image processing
-Atomic model buiding 1
| Initial model | PDB ID: Chain - Chain ID: a / Chain - Residue range: 33-1271 / Chain - Source name: PDB / Chain - Initial model type: experimental model |
|---|---|
| Refinement | Protocol: FLEXIBLE FIT |
| Output model |  PDB-6gdi:  PDB-6q81: |
 Movie
Movie Controller
Controller
















 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)