[English] 日本語
 Yorodumi
Yorodumi- EMDB-4310: Subtomogram average of OST-free ribosome-translocon complexes fro... -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: EMDB / ID: EMD-4310 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | Subtomogram average of OST-free ribosome-translocon complexes from STT3A(-/-) HEK cells | |||||||||
 Map data Map data | Subtomogram average of OST-free ribosome-translocon complexes from STT3A(-/-) HEK cells | |||||||||
 Sample Sample |
| |||||||||
| Function / homology |  Function and homology information Function and homology informationoligosaccharyltransferase complex A / oligosaccharyltransferase complex B / Insertion of tail-anchored proteins into the endoplasmic reticulum membrane / membrane docking / endoplasmic reticulum Sec complex / pronephric nephron development / dolichyl-diphosphooligosaccharide-protein glycotransferase / dolichyl-diphosphooligosaccharide-protein glycotransferase activity / cotranslational protein targeting to membrane / Ssh1 translocon complex ...oligosaccharyltransferase complex A / oligosaccharyltransferase complex B / Insertion of tail-anchored proteins into the endoplasmic reticulum membrane / membrane docking / endoplasmic reticulum Sec complex / pronephric nephron development / dolichyl-diphosphooligosaccharide-protein glycotransferase / dolichyl-diphosphooligosaccharide-protein glycotransferase activity / cotranslational protein targeting to membrane / Ssh1 translocon complex / Sec61 translocon complex / protein targeting to ER / protein insertion into ER membrane / protein-transporting ATPase activity / oligosaccharyltransferase complex / : / SRP-dependent cotranslational protein targeting to membrane, translocation / : / signal sequence binding / ribosomal subunit / post-translational protein targeting to membrane, translocation / : / protein transmembrane transporter activity / ubiquitin ligase inhibitor activity / positive regulation of signal transduction by p53 class mediator / rough endoplasmic reticulum / MDM2/MDM4 family protein binding / post-translational protein modification / cytosolic ribosome / guanyl-nucleotide exchange factor activity / ribosomal large subunit biogenesis / maturation of LSU-rRNA from tricistronic rRNA transcript (SSU-rRNA, 5.8S rRNA, LSU-rRNA) / phospholipid binding / regulation of translation / large ribosomal subunit / ribosome biogenesis / ribosome binding / 5S rRNA binding / ribosomal large subunit assembly / large ribosomal subunit rRNA binding / cytosolic large ribosomal subunit / cytoplasmic translation / tRNA binding / rRNA binding / structural constituent of ribosome / ribosome / translation / ribonucleoprotein complex / mRNA binding / synapse / endoplasmic reticulum membrane / nucleolus / endoplasmic reticulum / RNA binding / zinc ion binding / metal ion binding / nucleus / membrane / cytosol / cytoplasm Similarity search - Function | |||||||||
| Biological species |  Homo sapiens (human) Homo sapiens (human) | |||||||||
| Method | subtomogram averaging / cryo EM / Resolution: 30.0 Å | |||||||||
 Authors Authors | Pfeffer S / Foerster F | |||||||||
 Citation Citation |  Journal: Science / Year: 2018 Journal: Science / Year: 2018Title: Structural basis for coupling protein transport and N-glycosylation at the mammalian endoplasmic reticulum. Authors: Katharina Braunger / Stefan Pfeffer / Shiteshu Shrimal / Reid Gilmore / Otto Berninghausen / Elisabet C Mandon / Thomas Becker / Friedrich Förster / Roland Beckmann /    Abstract: Protein synthesis, transport, and N-glycosylation are coupled at the mammalian endoplasmic reticulum by complex formation of a ribosome, the Sec61 protein-conducting channel, and ...Protein synthesis, transport, and N-glycosylation are coupled at the mammalian endoplasmic reticulum by complex formation of a ribosome, the Sec61 protein-conducting channel, and oligosaccharyltransferase (OST). Here we used different cryo-electron microscopy approaches to determine structures of native and solubilized ribosome-Sec61-OST complexes. A molecular model for the catalytic OST subunit STT3A (staurosporine and temperature sensitive 3A) revealed how it is integrated into the OST and how STT3-paralog specificity for translocon-associated OST is achieved. The OST subunit DC2 was placed at the interface between Sec61 and STT3A, where it acts as a versatile module for recruitment of STT3A-containing OST to the ribosome-Sec61 complex. This detailed structural view on the molecular architecture of the cotranslational machinery for N-glycosylation provides the basis for a mechanistic understanding of glycoprotein biogenesis at the endoplasmic reticulum. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Movie |
 Movie viewer Movie viewer |
|---|---|
| Structure viewer | EM map:  SurfView SurfView Molmil Molmil Jmol/JSmol Jmol/JSmol |
| Supplemental images |
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_4310.map.gz emd_4310.map.gz | 4.7 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-4310-v30.xml emd-4310-v30.xml emd-4310.xml emd-4310.xml | 11.9 KB 11.9 KB | Display Display |  EMDB header EMDB header |
| Images |  emd_4310.png emd_4310.png | 50.4 KB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-4310 http://ftp.pdbj.org/pub/emdb/structures/EMD-4310 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-4310 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-4310 | HTTPS FTP |
-Related structure data
| Related structure data |  4306C  4307C  4308C  4309C  4311C  4312C  4313C  4314C  4315C  4316C  4317C  6ftgC  6ftiC  6ftjC C: citing same article ( |
|---|---|
| Similar structure data |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_4310.map.gz / Format: CCP4 / Size: 5.1 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_4310.map.gz / Format: CCP4 / Size: 5.1 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Subtomogram average of OST-free ribosome-translocon complexes from STT3A(-/-) HEK cells | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 5.24 Å | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
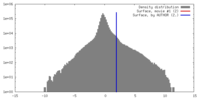
| Density |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
CCP4 map header:
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
-Supplemental data
- Sample components
Sample components
-Entire : 80S ribosome from STT3A(-/-) HEK cells bound to the endoplasmic r...
| Entire | Name: 80S ribosome from STT3A(-/-) HEK cells bound to the endoplasmic reticulum protein translocon |
|---|---|
| Components |
|
-Supramolecule #1: 80S ribosome from STT3A(-/-) HEK cells bound to the endoplasmic r...
| Supramolecule | Name: 80S ribosome from STT3A(-/-) HEK cells bound to the endoplasmic reticulum protein translocon type: complex / ID: 1 / Parent: 0 |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
-Supramolecule #2: OST-free protein translocon complexes of the endoplasmic reticulum
| Supramolecule | Name: OST-free protein translocon complexes of the endoplasmic reticulum type: complex / ID: 2 / Parent: 1 |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | subtomogram averaging |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Buffer | pH: 7.6 |
|---|---|
| Vitrification | Cryogen name: ETHANE-PROPANE |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Image recording | Film or detector model: GATAN K2 SUMMIT (4k x 4k) / Detector mode: COUNTING / Average electron dose: 1.5 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
- Image processing
Image processing
| Final reconstruction | Resolution.type: BY AUTHOR / Resolution: 30.0 Å / Resolution method: FSC 0.5 CUT-OFF / Number subtomograms used: 525 |
|---|---|
| Extraction | Number tomograms: 77 / Number images used: 793 / Software - Name: PyTom |
| Final angle assignment | Type: NOT APPLICABLE |
 Movie
Movie Controller
Controller
















 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)