[English] 日本語
 Yorodumi
Yorodumi- EMDB-42934: Focused classification of dsDNA nucleic acid density from in vitr... -
+ Open data
Open data
- Basic information
Basic information
| Entry |  | |||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Title | Focused classification of dsDNA nucleic acid density from in vitro CHMP1B/IST1 copolymer filaments - Class2 | |||||||||||||||||||||
 Map data Map data | Refined sharpened map | |||||||||||||||||||||
 Sample Sample |
| |||||||||||||||||||||
 Keywords Keywords | nucleic acid binding / DNA BINDING PROTEIN | |||||||||||||||||||||
| Function / homology |  Function and homology information Function and homology informationMIT domain binding / multivesicular body-lysosome fusion / amphisome membrane / vesicle fusion with vacuole / ESCRT III complex disassembly / late endosome to lysosome transport / ESCRT III complex / kinetochore microtubule / endosome transport via multivesicular body sorting pathway / cytoskeleton-dependent cytokinesis ...MIT domain binding / multivesicular body-lysosome fusion / amphisome membrane / vesicle fusion with vacuole / ESCRT III complex disassembly / late endosome to lysosome transport / ESCRT III complex / kinetochore microtubule / endosome transport via multivesicular body sorting pathway / cytoskeleton-dependent cytokinesis / collateral sprouting / membrane coat / nuclear membrane reassembly / Sealing of the nuclear envelope (NE) by ESCRT-III / positive regulation of collateral sprouting / multivesicular body sorting pathway / regulation of centrosome duplication / midbody abscission / membrane fission / plasma membrane repair / late endosome to vacuole transport / ubiquitin-dependent protein catabolic process via the multivesicular body sorting pathway / multivesicular body assembly / multivesicular body membrane / Flemming body / regulation of mitotic spindle assembly / mitotic metaphase chromosome alignment / nucleus organization / endoplasmic reticulum-Golgi intermediate compartment / viral budding via host ESCRT complex / autophagosome membrane / positive regulation of proteolysis / autophagosome maturation / nuclear pore / multivesicular body / viral budding from plasma membrane / establishment of protein localization / kinetochore / autophagy / azurophil granule lumen / intracellular protein localization / nuclear envelope / protein transport / midbody / endosome membrane / cadherin binding / intracellular membrane-bounded organelle / protein domain specific binding / cell division / lysosomal membrane / Neutrophil degranulation / centrosome / chromatin / protein-containing complex binding / extracellular exosome / extracellular region / nucleoplasm / identical protein binding / plasma membrane / cytosol Similarity search - Function | |||||||||||||||||||||
| Biological species |  Homo sapiens (human) Homo sapiens (human) | |||||||||||||||||||||
| Method | single particle reconstruction / cryo EM / Resolution: 2.99 Å | |||||||||||||||||||||
 Authors Authors | Talledge N / Laughlin TG / Alian A / McCullough J | |||||||||||||||||||||
| Funding support |  United States, 6 items United States, 6 items
| |||||||||||||||||||||
 Citation Citation |  Journal: To Be Published Journal: To Be PublishedTitle: ESCRT-III Assembles Around Mis-segregated DNA to Engage NoCut Authors: Talledge N / Glover J / McCullough J / Alian A / Sadler JBA / Dempsey N / Laughlin TG / Nguyen HC / Wenzel D / Lalonde MS / Ventimiglia LN / LaJoie D / Iwasa J / Starling T / Padilla-Parra S ...Authors: Talledge N / Glover J / McCullough J / Alian A / Sadler JBA / Dempsey N / Laughlin TG / Nguyen HC / Wenzel D / Lalonde MS / Ventimiglia LN / LaJoie D / Iwasa J / Starling T / Padilla-Parra S / Ullman KS / Frost A / Sundquist WI / Martin-Serrano J | |||||||||||||||||||||
| History |
|
- Structure visualization
Structure visualization
| Supplemental images |
|---|
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_42934.map.gz emd_42934.map.gz | 230.1 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-42934-v30.xml emd-42934-v30.xml emd-42934.xml emd-42934.xml | 24.2 KB 24.2 KB | Display Display |  EMDB header EMDB header |
| FSC (resolution estimation) |  emd_42934_fsc.xml emd_42934_fsc.xml | 13.2 KB | Display |  FSC data file FSC data file |
| Images |  emd_42934.png emd_42934.png | 97.9 KB | ||
| Masks |  emd_42934_msk_1.map emd_42934_msk_1.map | 244.1 MB |  Mask map Mask map | |
| Filedesc metadata |  emd-42934.cif.gz emd-42934.cif.gz | 6.4 KB | ||
| Others |  emd_42934_additional_1.map.gz emd_42934_additional_1.map.gz emd_42934_half_map_1.map.gz emd_42934_half_map_1.map.gz emd_42934_half_map_2.map.gz emd_42934_half_map_2.map.gz | 122 MB 226.5 MB 226.5 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-42934 http://ftp.pdbj.org/pub/emdb/structures/EMD-42934 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-42934 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-42934 | HTTPS FTP |
-Related structure data
| Related structure data |  8v2qC  8v2rC  8v2sC C: citing same article ( |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|
- Map
Map
| File |  Download / File: emd_42934.map.gz / Format: CCP4 / Size: 244.1 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_42934.map.gz / Format: CCP4 / Size: 244.1 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Refined sharpened map | ||||||||||||||||||||||||||||||||||||
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 0.8936 Å | ||||||||||||||||||||||||||||||||||||
| Density |
| ||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
|
-Supplemental data
-Mask #1
| File |  emd_42934_msk_1.map emd_42934_msk_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
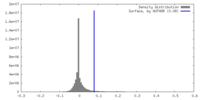
| Density Histograms |
-Additional map: Refined map
| File | emd_42934_additional_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Refined map | ||||||||||||
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Half map: Refined half map B
| File | emd_42934_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Refined half map B | ||||||||||||
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Half map: Refined half map A
| File | emd_42934_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Refined half map A | ||||||||||||
| Projections & Slices |
| ||||||||||||
| Density Histograms |
- Sample components
Sample components
-Entire : CHMP1B/IST1 copolymer bound to a 60-mer oligonucleotide of ssDNA
| Entire | Name: CHMP1B/IST1 copolymer bound to a 60-mer oligonucleotide of ssDNA |
|---|---|
| Components |
|
-Supramolecule #1: CHMP1B/IST1 copolymer bound to a 60-mer oligonucleotide of ssDNA
| Supramolecule | Name: CHMP1B/IST1 copolymer bound to a 60-mer oligonucleotide of ssDNA type: complex / ID: 1 / Parent: 0 / Macromolecule list: all Details: Complex assembly formed my mixing protein and oligonucleotide at a 1:20 molar ratio (protein to base) by dialysis into physiological buffer conditions |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 397.494 KDa |
-Supramolecule #2: Charged multivesicular body protein 1B (CHMP1B)
| Supramolecule | Name: Charged multivesicular body protein 1B (CHMP1B) / type: complex / ID: 2 / Parent: 1 / Macromolecule list: #1 Details: CHMP1B component of the nucleic acid templated helical assembly |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
-Supramolecule #3: Increased sodium tolerance 1 (IST1)
| Supramolecule | Name: Increased sodium tolerance 1 (IST1) / type: complex / ID: 3 / Parent: 1 / Macromolecule list: #2 Details: IST1 component of the nucleic acid templated assembly |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
-Macromolecule #1: Charged multivesicular body protein 1B (CHMP1B)
| Macromolecule | Name: Charged multivesicular body protein 1B (CHMP1B) / type: protein_or_peptide / ID: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Recombinant expression | Organism:  |
| Sequence | String: MSNMEKHLFN LKFAAKELSR SAKKCDKEEK AEKAKIKKAI QKGNMEVARI HAENAIRQKN QAVNFLRMS ARVDAVAARV QTAVTMGKVT KSMAGVVKSM DATLKTMNLE KISALMDKFE H QFETLDVQ TQQMEDTVSS TTTLTTPQNQ VDMLLQEMAD EAGLDLNMEL ...String: MSNMEKHLFN LKFAAKELSR SAKKCDKEEK AEKAKIKKAI QKGNMEVARI HAENAIRQKN QAVNFLRMS ARVDAVAARV QTAVTMGKVT KSMAGVVKSM DATLKTMNLE KISALMDKFE H QFETLDVQ TQQMEDTVSS TTTLTTPQNQ VDMLLQEMAD EAGLDLNMEL PQGQTGSVGT SV ASAEQDE LSQRLARLRD QV UniProtKB: Charged multivesicular body protein 1b |
-Macromolecule #2: Increased sodium tolerance 1 (IST1)
| Macromolecule | Name: Increased sodium tolerance 1 (IST1) / type: protein_or_peptide / ID: 2 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Recombinant expression | Organism:  |
| Sequence | String: MLGSGFKAER LRVNLRLVIN RLKLLEKKKT ELAQKARKEI ADYLAAGKDE RARIRVEHII REDYLVEAM EILELYCDLL LARFGLIQSM KELDSGLAES VSTLIWAAPR LQSEVAELKI V ADQLCAKY SKEYGKLCRT NQIGTVNDRL MHKLSVEAPP KILVERYLIE ...String: MLGSGFKAER LRVNLRLVIN RLKLLEKKKT ELAQKARKEI ADYLAAGKDE RARIRVEHII REDYLVEAM EILELYCDLL LARFGLIQSM KELDSGLAES VSTLIWAAPR LQSEVAELKI V ADQLCAKY SKEYGKLCRT NQIGTVNDRL MHKLSVEAPP KILVERYLIE IAKNYNVPYE PD SVVMAEA PPGVETDLID VGFTDDVKKG GPGRGGSGGF TAPVGGPDGT VPMPMPMPMP SAN TPFSYP LPKGPSDFNG LPMGTYQAFP NIHPPQIPAT PPSYESVDDI NADKNISSAQ IVGP GPKPE ASAKLPSRPA DNYDNFVLPE LPSVPDTLPT ASAGASTSAS EDIDFDDLSR RFEEL KKKT UniProtKB: IST1 homolog |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | filament |
- Sample preparation
Sample preparation
| Buffer | pH: 8 Component:
| |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Vitrification | Cryogen name: ETHANE / Chamber humidity: 100 % / Chamber temperature: 292.15 K / Instrument: FEI VITROBOT MARK IV | |||||||||
| Details | Sample is at 16 micromolar protein concentration. |
- Electron microscopy
Electron microscopy
| Microscope | TFS GLACIOS |
|---|---|
| Specialist optics | Energy filter - Name: TFS Selectris / Energy filter - Slit width: 10 eV |
| Image recording | Film or detector model: FEI FALCON IV (4k x 4k) / Number grids imaged: 1 / Average electron dose: 50.0 e/Å2 |
| Electron beam | Acceleration voltage: 200 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | C2 aperture diameter: 50.0 µm / Calibrated defocus max: 4.0 µm / Calibrated defocus min: 0.1 µm / Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD / Nominal defocus max: 1.8 µm / Nominal defocus min: 0.8 µm / Nominal magnification: 130000 |
| Sample stage | Specimen holder model: OTHER / Cooling holder cryogen: NITROGEN |
+ Image processing
Image processing
-Atomic model buiding 1
| Initial model |
| |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Refinement | Space: REAL / Protocol: OTHER |
 Movie
Movie Controller
Controller

















 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)