[English] 日本語
 Yorodumi
Yorodumi- EMDB-42228: High resolution in-situ structure of complex III in respiratory s... -
+ Open data
Open data
- Basic information
Basic information
| Entry |  | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | High resolution in-situ structure of complex III in respiratory supercomplex (composite) | |||||||||
 Map data Map data | ||||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | in-situ cryo-EM structure / mammalian / mitochondria / respiratory supercomplex / proton pumping / membrane protein / electron transport | |||||||||
| Function / homology |  Function and homology information Function and homology informationComplex III assembly / subthalamus development / pons development / cerebellar Purkinje cell layer development / Respiratory electron transport / pyramidal neuron development / thalamus development / Mitochondrial protein degradation / respiratory chain complex / respiratory chain complex III ...Complex III assembly / subthalamus development / pons development / cerebellar Purkinje cell layer development / Respiratory electron transport / pyramidal neuron development / thalamus development / Mitochondrial protein degradation / respiratory chain complex / respiratory chain complex III / quinol-cytochrome-c reductase / quinol-cytochrome-c reductase activity / mitochondrial electron transport, ubiquinol to cytochrome c / hypothalamus development / midbrain development / hippocampus development / metalloendopeptidase activity / 2 iron, 2 sulfur cluster binding / electron transfer activity / mitochondrial inner membrane / heme binding / protein-containing complex / mitochondrion / proteolysis / nucleoplasm / metal ion binding / membrane Similarity search - Function | |||||||||
| Biological species |  | |||||||||
| Method | single particle reconstruction / cryo EM / Resolution: 2.1 Å | |||||||||
 Authors Authors | Zheng W / Zhu J / Zhang K | |||||||||
| Funding support |  United States, 1 items United States, 1 items
| |||||||||
 Citation Citation |  Journal: Nature / Year: 2024 Journal: Nature / Year: 2024Title: High-resolution in situ structures of mammalian respiratory supercomplexes. Authors: Wan Zheng / Pengxin Chai / Jiapeng Zhu / Kai Zhang /   Abstract: Mitochondria play a pivotal part in ATP energy production through oxidative phosphorylation, which occurs within the inner membrane through a series of respiratory complexes. Despite extensive in ...Mitochondria play a pivotal part in ATP energy production through oxidative phosphorylation, which occurs within the inner membrane through a series of respiratory complexes. Despite extensive in vitro structural studies, determining the atomic details of their molecular mechanisms in physiological states remains a major challenge, primarily because of loss of the native environment during purification. Here we directly image porcine mitochondria using an in situ cryo-electron microscopy approach. This enables us to determine the structures of various high-order assemblies of respiratory supercomplexes in their native states. We identify four main supercomplex organizations: IIIIIV, IIIIIV, IIIIIV and IIIIIV, which potentially expand into higher-order arrays on the inner membranes. These diverse supercomplexes are largely formed by 'protein-lipids-protein' interactions, which in turn have a substantial impact on the local geometry of the surrounding membranes. Our in situ structures also capture numerous reactive intermediates within these respiratory supercomplexes, shedding light on the dynamic processes of the ubiquinone/ubiquinol exchange mechanism in complex I and the Q-cycle in complex III. Structural comparison of supercomplexes from mitochondria treated under different conditions indicates a possible correlation between conformational states of complexes I and III, probably in response to environmental changes. By preserving the native membrane environment, our approach enables structural studies of mitochondrial respiratory supercomplexes in reaction at high resolution across multiple scales, from atomic-level details to the broader subcellular context. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Supplemental images |
|---|
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_42228.map.gz emd_42228.map.gz | 386.8 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-42228-v30.xml emd-42228-v30.xml emd-42228.xml emd-42228.xml | 24.8 KB 24.8 KB | Display Display |  EMDB header EMDB header |
| Images |  emd_42228.png emd_42228.png | 72.7 KB | ||
| Filedesc metadata |  emd-42228.cif.gz emd-42228.cif.gz | 7.5 KB | ||
| Others |  emd_42228_half_map_1.map.gz emd_42228_half_map_1.map.gz emd_42228_half_map_2.map.gz emd_42228_half_map_2.map.gz | 6.7 MB 6.7 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-42228 http://ftp.pdbj.org/pub/emdb/structures/EMD-42228 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-42228 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-42228 | HTTPS FTP |
-Validation report
| Summary document |  emd_42228_validation.pdf.gz emd_42228_validation.pdf.gz | 621.9 KB | Display |  EMDB validaton report EMDB validaton report |
|---|---|---|---|---|
| Full document |  emd_42228_full_validation.pdf.gz emd_42228_full_validation.pdf.gz | 621.4 KB | Display | |
| Data in XML |  emd_42228_validation.xml.gz emd_42228_validation.xml.gz | 19.7 KB | Display | |
| Data in CIF |  emd_42228_validation.cif.gz emd_42228_validation.cif.gz | 22.3 KB | Display | |
| Arichive directory |  https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-42228 https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-42228 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-42228 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-42228 | HTTPS FTP |
-Related structure data
| Related structure data |  8ugkMC  8ud1C  8ueoC  8uepC  8ueqC  8uerC  8uesC  8uetC  8ueuC  8uevC  8uewC  8uexC  8ueyC  8uezC  8ugdC  8ugeC  8ugfC  8uggC  8ughC  8ugiC  8ugjC  8uglC  8ugnC  8ugpC  8ugrC M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_42228.map.gz / Format: CCP4 / Size: 518 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_42228.map.gz / Format: CCP4 / Size: 518 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 0.416 Å | ||||||||||||||||||||||||||||||||||||
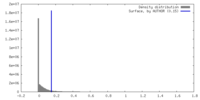
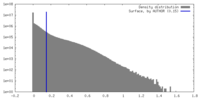
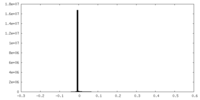
| Density |
| ||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
|
-Supplemental data
-Half map: #2
| File | emd_42228_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
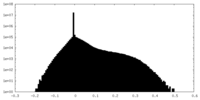
| Density Histograms |
-Half map: #1
| File | emd_42228_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
| Density Histograms |
- Sample components
Sample components
+Entire : In-situ cryo-EM structure of respiratory supercomplex by directly...
+Supramolecule #1: In-situ cryo-EM structure of respiratory supercomplex by directly...
+Macromolecule #1: Cytochrome b-c1 complex subunit 1, mitochondrial
+Macromolecule #2: Cytochrome b-c1 complex subunit 2, mitochondrial
+Macromolecule #3: Cytochrome b
+Macromolecule #4: Cytochrome c1, heme protein, mitochondrial
+Macromolecule #5: Cytochrome b-c1 complex subunit Rieske, mitochondrial
+Macromolecule #6: Cytochrome b-c1 complex subunit 7
+Macromolecule #7: Cytochrome b-c1 complex subunit 8
+Macromolecule #8: Cytochrome b-c1 complex subunit 6, mitochondrial
+Macromolecule #9: Ubiquinol-cytochrome c reductase complex 7.2 kDa protein
+Macromolecule #10: Cytochrome b-c1 complex subunit 10
+Macromolecule #11: CARDIOLIPIN
+Macromolecule #12: 1,2-Distearoyl-sn-glycerophosphoethanolamine
+Macromolecule #13: PROTOPORPHYRIN IX CONTAINING FE
+Macromolecule #14: HEME C
+Macromolecule #15: FE2/S2 (INORGANIC) CLUSTER
+Macromolecule #16: 1,2-DIACYL-SN-GLYCERO-3-PHOSPHOCHOLINE
+Macromolecule #17: water
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Buffer | pH: 7 |
|---|---|
| Vitrification | Cryogen name: ETHANE |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Image recording | Film or detector model: GATAN K3 BIOQUANTUM (6k x 4k) / Average electron dose: 50.0 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD / Cs: 2.7 mm / Nominal defocus max: 3.2 µm / Nominal defocus min: 1.0 µm |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
- Image processing
Image processing
| Startup model | Type of model: NONE |
|---|---|
| Final reconstruction | Resolution.type: BY AUTHOR / Resolution: 2.1 Å / Resolution method: FSC 0.143 CUT-OFF / Number images used: 300000 |
| Initial angle assignment | Type: MAXIMUM LIKELIHOOD |
| Final angle assignment | Type: MAXIMUM LIKELIHOOD |
 Movie
Movie Controller
Controller
































 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)