+ データを開く
データを開く
- 基本情報
基本情報
| 登録情報 | データベース: EMDB / ID: EMD-4190 | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| タイトル | A mechanism for the activation of the influenza virus transcriptase | ||||||||||||
 マップデータ マップデータ | EM map of influenza C virus RNA dependent RNA polymerase bound to promoter vRNA | ||||||||||||
 試料 試料 |
| ||||||||||||
 キーワード キーワード | influenza virus RNA dependent RNA polymerase / VIRAL PROTEIN | ||||||||||||
| 機能・相同性 |  機能・相同性情報 機能・相同性情報cap snatching / viral transcription / symbiont-mediated suppression of host mRNA transcription via inhibition of RNA polymerase II activity / 7-methylguanosine mRNA capping / host cell mitochondrion / virion component / endonuclease activity / 加水分解酵素; エステル加水分解酵素 / host cell cytoplasm / symbiont-mediated suppression of host gene expression ...cap snatching / viral transcription / symbiont-mediated suppression of host mRNA transcription via inhibition of RNA polymerase II activity / 7-methylguanosine mRNA capping / host cell mitochondrion / virion component / endonuclease activity / 加水分解酵素; エステル加水分解酵素 / host cell cytoplasm / symbiont-mediated suppression of host gene expression / viral translational frameshifting / RNA-directed RNA polymerase / viral RNA genome replication / nucleotide binding / RNA-directed RNA polymerase activity / DNA-templated transcription / host cell nucleus / RNA binding / metal ion binding 類似検索 - 分子機能 | ||||||||||||
| 生物種 |  Influenza B virus (B型インフルエンザウイルス) / Influenza B virus (B型インフルエンザウイルス) /  Influenza B virus (B/Memphis/13/2003) (B型インフルエンザウイルス) Influenza B virus (B/Memphis/13/2003) (B型インフルエンザウイルス) | ||||||||||||
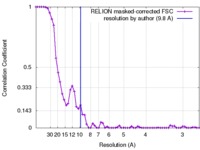
| 手法 | 単粒子再構成法 / クライオ電子顕微鏡法 / 解像度: 9.8 Å | ||||||||||||
 データ登録者 データ登録者 | Serna Martin I / Grimes JM | ||||||||||||
| 資金援助 |  英国, 3件 英国, 3件
| ||||||||||||
 引用 引用 |  ジャーナル: Mol Cell / 年: 2018 ジャーナル: Mol Cell / 年: 2018タイトル: A Mechanism for the Activation of the Influenza Virus Transcriptase. 著者: Itziar Serna Martin / Narin Hengrung / Max Renner / Jane Sharps / Mónica Martínez-Alonso / Simonas Masiulis / Jonathan M Grimes / Ervin Fodor /  要旨: Influenza virus RNA polymerase (FluPol), a heterotrimer composed of PB1, PB2, and PA subunits (P3 in influenza C), performs both transcription and replication of the viral RNA genome. For ...Influenza virus RNA polymerase (FluPol), a heterotrimer composed of PB1, PB2, and PA subunits (P3 in influenza C), performs both transcription and replication of the viral RNA genome. For transcription, FluPol interacts with the C-terminal domain (CTD) of RNA polymerase II (Pol II), which enables FluPol to snatch capped RNA primers from nascent host RNAs. Here, we describe the co-crystal structure of influenza C virus polymerase (FluPol) bound to a Ser5-phosphorylated CTD (pS-CTD) peptide. The position of the CTD-binding site at the interface of PB1, P3, and the flexible PB2 C-terminal domains suggests that CTD binding stabilizes the transcription-competent conformation of FluPol. In agreement, both cap snatching and capped primer-dependent transcription initiation by FluPol are enhanced in the presence of pS-CTD. Mutations of amino acids in the CTD-binding site reduce viral mRNA synthesis. We propose a model for the activation of the influenza virus transcriptase through its association with pS-CTD of Pol II. | ||||||||||||
| 履歴 |
|
- 構造の表示
構造の表示
| ムービー |
 ムービービューア ムービービューア |
|---|---|
| 構造ビューア | EMマップ:  SurfView SurfView Molmil Molmil Jmol/JSmol Jmol/JSmol |
| 添付画像 |
- ダウンロードとリンク
ダウンロードとリンク
-EMDBアーカイブ
| マップデータ |  emd_4190.map.gz emd_4190.map.gz | 2 MB |  EMDBマップデータ形式 EMDBマップデータ形式 | |
|---|---|---|---|---|
| ヘッダ (付随情報) |  emd-4190-v30.xml emd-4190-v30.xml emd-4190.xml emd-4190.xml | 15.9 KB 15.9 KB | 表示 表示 |  EMDBヘッダ EMDBヘッダ |
| FSC (解像度算出) |  emd_4190_fsc.xml emd_4190_fsc.xml | 5.5 KB | 表示 |  FSCデータファイル FSCデータファイル |
| 画像 |  emd_4190.png emd_4190.png | 57 KB | ||
| Filedesc metadata |  emd-4190.cif.gz emd-4190.cif.gz | 6.9 KB | ||
| アーカイブディレクトリ |  http://ftp.pdbj.org/pub/emdb/structures/EMD-4190 http://ftp.pdbj.org/pub/emdb/structures/EMD-4190 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-4190 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-4190 | HTTPS FTP |
-検証レポート
| 文書・要旨 |  emd_4190_validation.pdf.gz emd_4190_validation.pdf.gz | 233 KB | 表示 |  EMDB検証レポート EMDB検証レポート |
|---|---|---|---|---|
| 文書・詳細版 |  emd_4190_full_validation.pdf.gz emd_4190_full_validation.pdf.gz | 232.2 KB | 表示 | |
| XML形式データ |  emd_4190_validation.xml.gz emd_4190_validation.xml.gz | 8.4 KB | 表示 | |
| アーカイブディレクトリ |  https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-4190 https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-4190 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-4190 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-4190 | HTTPS FTP |
-関連構造データ
- リンク
リンク
| EMDBのページ |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|
- マップ
マップ
| ファイル |  ダウンロード / ファイル: emd_4190.map.gz / 形式: CCP4 / 大きさ: 15.6 MB / タイプ: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) ダウンロード / ファイル: emd_4190.map.gz / 形式: CCP4 / 大きさ: 15.6 MB / タイプ: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 注釈 | EM map of influenza C virus RNA dependent RNA polymerase bound to promoter vRNA | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 投影像・断面図 | 画像のコントロール
画像は Spider により作成 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| ボクセルのサイズ | X=Y=Z: 1.35 Å | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 密度 |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 対称性 | 空間群: 1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 詳細 | EMDB XML:
CCP4マップ ヘッダ情報:
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
-添付データ
- 試料の構成要素
試料の構成要素
-全体 : Influenza C virus polymerase bound to promoter vRNA
| 全体 | 名称: Influenza C virus polymerase bound to promoter vRNA |
|---|---|
| 要素 |
|
-超分子 #1: Influenza C virus polymerase bound to promoter vRNA
| 超分子 | 名称: Influenza C virus polymerase bound to promoter vRNA / タイプ: complex / ID: 1 / 親要素: 0 / 含まれる分子: all |
|---|---|
| 由来(天然) | 生物種:  Influenza B virus (B型インフルエンザウイルス) Influenza B virus (B型インフルエンザウイルス) |
-分子 #1: Polymerase acidic protein
| 分子 | 名称: Polymerase acidic protein / タイプ: protein_or_peptide / ID: 1 / コピー数: 1 / 光学異性体: LEVO |
|---|---|
| 由来(天然) | 生物種:  Influenza B virus (B型インフルエンザウイルス) Influenza B virus (B型インフルエンザウイルス) |
| 分子量 | 理論値: 83.232109 KDa |
| 組換発現 | 生物種:  |
| 配列 | 文字列: SMDTFITRNF QTTIIQKAKN TMAEFSEDPE LQPAMLFNIC VHLEVCYVIS DMNFLDEEGK AYTALEGQGK EQNLRPQYEV IEGMPRTIA WMVQRSLAQE HGIETPKYLA DLFDYKTKRF IEVGITKGLA DDYFWKKKEK LGNSMELMIF SYNQDYSLSN E SSLDEEGK ...文字列: SMDTFITRNF QTTIIQKAKN TMAEFSEDPE LQPAMLFNIC VHLEVCYVIS DMNFLDEEGK AYTALEGQGK EQNLRPQYEV IEGMPRTIA WMVQRSLAQE HGIETPKYLA DLFDYKTKRF IEVGITKGLA DDYFWKKKEK LGNSMELMIF SYNQDYSLSN E SSLDEEGK GRVLSRLTEL QAELSLKNLW QVLIGEEDVE KGIDFKLGQT ISRLRDISVP AGFSNFEGMR SYIDNIDPKG AI ERNLARM SPLVSVTPKK LTWEDLRPIG PHIYNHELPE VPYNAFLLMS DELGLANMTE GKSKKPKTLA KECLEKYSTL RDQ TDPILI MKSEKANENF LWKLWRDCVN TISNEEMSNE LQKTNYAKWA TGDGLTYQKI MKEVAIDDET MCQEEPKIPN KCRV AAWVQ TEMNLLSTLT SKRALDLPEI GPDVAPVEHV GSERRKYFVN EINYCKASTV MMKYVLFHTS LLNESNASMG KYKVI PITN RVVNEKGESF DMLYGLAVKG QSHLRGDTDV VTVVTFEFSS TDPRVDSGKW PKYTVFRIGS LFVSGREKSV YLYCRV NGT NKIQMKWGME ARRCLLQSMQ QMEAIVEQES SIQGYDMTKA CFKGDRVNSP KTFSIGTQEG KLVKGSFGKA LRVIFTK CL MHYVFGNAQL EGFSAESRRL LLLIQALKDR KGPWVFDLEG MYSGIEECIS NNPWVIQSAY WFNEWLGFEK EGSKVLES V DEIMDE UniProtKB: Polymerase acidic protein |
-分子 #2: RNA-directed RNA polymerase catalytic subunit
| 分子 | 名称: RNA-directed RNA polymerase catalytic subunit / タイプ: protein_or_peptide / ID: 2 / コピー数: 1 / 光学異性体: LEVO / EC番号: RNA-directed RNA polymerase |
|---|---|
| 由来(天然) | 生物種:  Influenza B virus (B型インフルエンザウイルス) Influenza B virus (B型インフルエンザウイルス) |
| 分子量 | 理論値: 84.433266 KDa |
| 組換発現 | 生物種:  |
| 配列 | 文字列: MNINPYFLFI DVPIQAAIST TFPYTGVPPY SHGTGTGYTI DTVIRTHEYS NKGKQYISDV TGCTMVDPTN GPLPEDNEPS AYAQLDCVL EALDRMDEEH PGLFQAASQN AMETLMVTTV DKLTQGRQTF DWTVCRNQPA ATALNTTITS FRLNDLNGAD K GGLIPFCQ ...文字列: MNINPYFLFI DVPIQAAIST TFPYTGVPPY SHGTGTGYTI DTVIRTHEYS NKGKQYISDV TGCTMVDPTN GPLPEDNEPS AYAQLDCVL EALDRMDEEH PGLFQAASQN AMETLMVTTV DKLTQGRQTF DWTVCRNQPA ATALNTTITS FRLNDLNGAD K GGLIPFCQ DIIDSLDRPE MTFFSVKNIK KKLPAKNRKG FLIKRIPMKV KDKITKVEYI KRALSLNTMT KDAERGKLKR RA IATAGIQ IRGFVLVVEN LAKNICENLE QSGLPVGGNE KKAKLSNAVA KMLSNCPPGG ISMTVTGDNT KWNECLNPRI FLA MTERIT RDSPIWFRDF CSIAPVLFSN KIARLGKGFM ITSKTKRLKA QIPCPDLFSI PLERYNEETR AKLKKLKPFF NEEG TASLS PGMMMGMFNM LSTVLGVAAL GIKNIGNKEY LWDGLQSSDD FALFVNAKDE ETCMEGINDF YRTCKLLGIN MSKKK SYCN ETGMFEFTSM FYRDGFVSNF AMELPSFGVA GVNESADMAI GMTIIKNNMI NNGMGPATAQ TAIQLFIADY RYTYKC HRG DSKVEGKRMK IIKELWENTK GRDGLLVADG GPNIYNLRNL HIPEIVLKYN LMDPEYKGRL LHPQNPFVGH LSIEGIK EA DITPAHGPVK KMDYDAVSGT HSWRTKRNRS ILNTDQRNMI LEEQCYAKCC NLFEACFNSA SYRKPVGQHS MLEAMAHR L RMDARLDYES GRMSKDDFEK AMAHLGEIGY I UniProtKB: RNA-directed RNA polymerase catalytic subunit |
-分子 #3: Polymerase basic protein 2
| 分子 | 名称: Polymerase basic protein 2 / タイプ: protein_or_peptide / ID: 3 / コピー数: 1 / 光学異性体: LEVO |
|---|---|
| 由来(天然) | 生物種:  Influenza B virus (B型インフルエンザウイルス) Influenza B virus (B型インフルエンザウイルス) |
| 分子量 | 理論値: 88.028211 KDa |
| 組換発現 | 生物種:  |
| 配列 | 文字列: MTLAKIELLK QLLRDNEAKT VLKQTTVDQY NIIRKFNTSR IEKNPSLRMK WAMCSNFPLA LTKGDMANRI PLEYKGIQLK TNAEDIGTK GQMCSIAAVT WWNTYGPIGD TEGFERVYES FFLRKMRLDN ATWGRITFGP VERVRKRVLL NPLTKEMPPD E ASNVIMEI ...文字列: MTLAKIELLK QLLRDNEAKT VLKQTTVDQY NIIRKFNTSR IEKNPSLRMK WAMCSNFPLA LTKGDMANRI PLEYKGIQLK TNAEDIGTK GQMCSIAAVT WWNTYGPIGD TEGFERVYES FFLRKMRLDN ATWGRITFGP VERVRKRVLL NPLTKEMPPD E ASNVIMEI LFPKEAGIPR ESTWIHRELI KEKREKLKGT MITPIVLAYM LERELVARRR FLPVAGATSA EFIEMLHCLQ GE NWRQIYH PGGNKLTESR SQSMIVACRK IIRRSIVASN PLELAVEIAN KTVIDTEPLK SCLAAIDGGD VACDIIRAAL GLK IRQRQR FGRLELKRIS GRGFKNDEEI LIGNGTIQKI GIWDGEEEFH VRCGECRGIL KKSKMKLEKL LINSAKKEDM RDLI ILCMV FSQDTRMFQG VRGEINFLNR AGQLLSPMYQ LQRYFLNRSN DLFDQWGYEE SPKASELHGI NESMNASDYT LKGVV VTRN VIDDFSSTET EKVSITKNLS LIKRTGEVIM GANDVSELES QAQLMITYDT PKMWEMGTTK ELVQNTYQWV LKNLVT LKA QFLLGKEDMF QWDAFEAFES IIPQKMAGQY SGFARAVLKQ MRDQEVMKTD QFIKLLPFCF SPPKLRSNGE PYQFLKL VL KGGGENFIEV RKGSPLFSYN PQTEVLTICG RMMSLKGKIE DEERNRSMGN AVLAGFLVSG KYDPDLGDFK TIEELEKL K PGEKANILLY QGKPVKVVKR KRYSALSNDI SQGIKRQRMT VESMGWALS UniProtKB: Polymerase basic protein 2 |
-分子 #4: 3' promoter vRNA
| 分子 | 名称: 3' promoter vRNA / タイプ: rna / ID: 4 / コピー数: 1 |
|---|---|
| 由来(天然) | 生物種:  Influenza B virus (B/Memphis/13/2003) (B型インフルエンザウイルス) Influenza B virus (B/Memphis/13/2003) (B型インフルエンザウイルス) |
| 分子量 | 理論値: 4.321563 KDa |
| 配列 | 文字列: UAUACCUCUG CUUC |
-分子 #5: 5' promoter vRNA
| 分子 | 名称: 5' promoter vRNA / タイプ: rna / ID: 5 / コピー数: 1 |
|---|---|
| 由来(天然) | 生物種:  Influenza B virus (B/Memphis/13/2003) (B型インフルエンザウイルス) Influenza B virus (B/Memphis/13/2003) (B型インフルエンザウイルス) |
| 分子量 | 理論値: 4.55782 KDa |
| 配列 | 文字列: AGUAGUAACA AGAG |
-実験情報
-構造解析
| 手法 | クライオ電子顕微鏡法 |
|---|---|
 解析 解析 | 単粒子再構成法 |
| 試料の集合状態 | particle |
- 試料調製
試料調製
| 緩衝液 | pH: 7.5 |
|---|---|
| 凍結 | 凍結剤: ETHANE |
- 電子顕微鏡法
電子顕微鏡法
| 顕微鏡 | FEI POLARA 300 |
|---|---|
| 撮影 | フィルム・検出器のモデル: GATAN K2 SUMMIT (4k x 4k) 平均電子線量: 2.09 e/Å2 |
| 電子線 | 加速電圧: 300 kV / 電子線源:  FIELD EMISSION GUN FIELD EMISSION GUN |
| 電子光学系 | 照射モード: FLOOD BEAM / 撮影モード: BRIGHT FIELD |
| 実験機器 |  モデル: Tecnai Polara / 画像提供: FEI Company |
+ 画像解析
画像解析
-原子モデル構築 1
| 精密化 | プロトコル: RIGID BODY FIT |
|---|---|
| 得られたモデル |  PDB-6f5o: |
 ムービー
ムービー コントローラー
コントローラー













 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)