[English] 日本語
 Yorodumi
Yorodumi- EMDB-4139: Cryo-EM reconstruction of the maedi-visna virus (MVV) strand tran... -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: EMDB / ID: EMD-4139 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | Cryo-EM reconstruction of the maedi-visna virus (MVV) strand transfer complex | |||||||||
 Map data Map data | MVV STC reconstruction at 8.6 %u212B resolution. | |||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | retrovirus / lentivirus / integrase / DNA-binding / Zn-binding / RNAseH fold / hydrolase | |||||||||
| Function / homology |  Function and homology information Function and homology informationdUTP diphosphatase / dUTP diphosphatase activity / nucleotide metabolic process / ribonuclease H / Hydrolases; Acting on peptide bonds (peptidases); Aspartic endopeptidases / exoribonuclease H / exoribonuclease H activity / DNA integration / viral genome integration into host DNA / establishment of integrated proviral latency ...dUTP diphosphatase / dUTP diphosphatase activity / nucleotide metabolic process / ribonuclease H / Hydrolases; Acting on peptide bonds (peptidases); Aspartic endopeptidases / exoribonuclease H / exoribonuclease H activity / DNA integration / viral genome integration into host DNA / establishment of integrated proviral latency / RNA-directed DNA polymerase / RNA stem-loop binding / RNA-directed DNA polymerase activity / RNA-DNA hybrid ribonuclease activity / Transferases; Transferring phosphorus-containing groups; Nucleotidyltransferases / viral capsid / DNA recombination / DNA-directed DNA polymerase / aspartic-type endopeptidase activity / Hydrolases; Acting on ester bonds / DNA-directed DNA polymerase activity / viral translational frameshifting / symbiont entry into host cell / proteolysis / DNA binding / zinc ion binding Similarity search - Function | |||||||||
| Biological species |  Maedi visna virus (strain KV1772) / Maedi visna virus (strain KV1772) /  Visna lentivirus (strain 1514) / synthetic construct (others) Visna lentivirus (strain 1514) / synthetic construct (others) | |||||||||
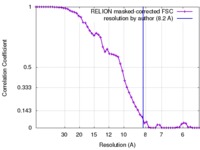
| Method | single particle reconstruction / cryo EM / Resolution: 8.2 Å | |||||||||
 Authors Authors | Pye VE / Ballandras-Colas A | |||||||||
| Funding support |  United States, 1 items United States, 1 items
| |||||||||
 Citation Citation |  Journal: Science / Year: 2017 Journal: Science / Year: 2017Title: A supramolecular assembly mediates lentiviral DNA integration. Authors: Allison Ballandras-Colas / Daniel P Maskell / Erik Serrao / Julia Locke / Paolo Swuec / Stefán R Jónsson / Abhay Kotecha / Nicola J Cook / Valerie E Pye / Ian A Taylor / Valgerdur ...Authors: Allison Ballandras-Colas / Daniel P Maskell / Erik Serrao / Julia Locke / Paolo Swuec / Stefán R Jónsson / Abhay Kotecha / Nicola J Cook / Valerie E Pye / Ian A Taylor / Valgerdur Andrésdóttir / Alan N Engelman / Alessandro Costa / Peter Cherepanov /    Abstract: Retroviral integrase (IN) functions within the intasome nucleoprotein complex to catalyze insertion of viral DNA into cellular chromatin. Using cryo-electron microscopy, we now visualize the ...Retroviral integrase (IN) functions within the intasome nucleoprotein complex to catalyze insertion of viral DNA into cellular chromatin. Using cryo-electron microscopy, we now visualize the functional maedi-visna lentivirus intasome at 4.9 angstrom resolution. The intasome comprises a homo-hexadecamer of IN with a tetramer-of-tetramers architecture featuring eight structurally distinct types of IN protomers supporting two catalytically competent subunits. The conserved intasomal core, previously observed in simpler retroviral systems, is formed between two IN tetramers, with a pair of C-terminal domains from flanking tetramers completing the synaptic interface. Our results explain how HIV-1 IN, which self-associates into higher-order multimers, can form a functional intasome, reconcile the bulk of early HIV-1 IN biochemical and structural data, and provide a lentiviral platform for design of HIV-1 IN inhibitors. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Movie |
 Movie viewer Movie viewer |
|---|---|
| Structure viewer | EM map:  SurfView SurfView Molmil Molmil Jmol/JSmol Jmol/JSmol |
| Supplemental images |
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_4139.map.gz emd_4139.map.gz | 1.2 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-4139-v30.xml emd-4139-v30.xml emd-4139.xml emd-4139.xml | 21.4 KB 21.4 KB | Display Display |  EMDB header EMDB header |
| FSC (resolution estimation) |  emd_4139_fsc.xml emd_4139_fsc.xml | 5.4 KB | Display |  FSC data file FSC data file |
| Images |  emd_4139.png emd_4139.png | 146.6 KB | ||
| Filedesc metadata |  emd-4139.cif.gz emd-4139.cif.gz | 6.6 KB | ||
| Others |  emd_4139_half_map_1.map.gz emd_4139_half_map_1.map.gz emd_4139_half_map_2.map.gz emd_4139_half_map_2.map.gz | 9 MB 9 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-4139 http://ftp.pdbj.org/pub/emdb/structures/EMD-4139 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-4139 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-4139 | HTTPS FTP |
-Related structure data
| Related structure data |  5m0rMC  5lljC  5t3aC  7zppC M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_4139.map.gz / Format: CCP4 / Size: 12.9 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_4139.map.gz / Format: CCP4 / Size: 12.9 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | MVV STC reconstruction at 8.6 %u212B resolution. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 2.7 Å | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
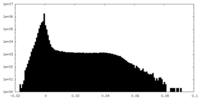
| Density |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
CCP4 map header:
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
-Supplemental data
-Half map: None
| File | emd_4139_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | None | ||||||||||||
| Projections & Slices |
| ||||||||||||
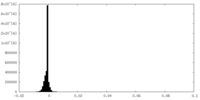
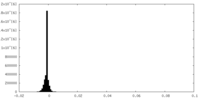
| Density Histograms |
-Half map: None
| File | emd_4139_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | None | ||||||||||||
| Projections & Slices |
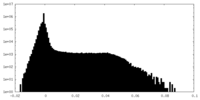
| ||||||||||||
| Density Histograms |
- Sample components
Sample components
-Entire : MVV strand transfer complex
| Entire | Name: MVV strand transfer complex |
|---|---|
| Components |
|
-Supramolecule #1: MVV strand transfer complex
| Supramolecule | Name: MVV strand transfer complex / type: complex / ID: 1 / Parent: 0 / Macromolecule list: all |
|---|
-Supramolecule #2: intergrase
| Supramolecule | Name: intergrase / type: complex / ID: 2 / Parent: 1 / Macromolecule list: #1 |
|---|---|
| Source (natural) | Organism:  Maedi visna virus (strain KV1772) Maedi visna virus (strain KV1772) |
-Supramolecule #3: nucleic acid
| Supramolecule | Name: nucleic acid / type: complex / ID: 3 / Parent: 1 / Macromolecule list: #2-#3 |
|---|---|
| Source (natural) | Organism:  Visna lentivirus (strain 1514) Visna lentivirus (strain 1514) |
-Supramolecule #4: nucleic acid
| Supramolecule | Name: nucleic acid / type: complex / ID: 4 / Parent: 1 / Macromolecule list: #4 |
|---|---|
| Source (natural) | Organism: synthetic construct (others) |
-Macromolecule #1: integrase
| Macromolecule | Name: integrase / type: protein_or_peptide / ID: 1 / Number of copies: 16 / Enantiomer: LEVO EC number: Hydrolases; Acting on peptide bonds (peptidases); Aspartic endopeptidases |
|---|---|
| Source (natural) | Organism:  Maedi visna virus (strain KV1772) Maedi visna virus (strain KV1772) |
| Molecular weight | Theoretical: 32.368826 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: WIENIPLAEE EHNKWHQDAV SLHLEFGIPR TAAEDIVQQC DVCQENKMPS TLRGSNKRGI DHWQVDYTHY EDKIILVWVE TNSGLIYAE RVKGETGQEF RVQTMKWYAM FAPKSLQSDN GPAFVAESTQ LLMKYLGIEH TTGIPWNPQS QALVERTHQT L KNTLEKLI ...String: WIENIPLAEE EHNKWHQDAV SLHLEFGIPR TAAEDIVQQC DVCQENKMPS TLRGSNKRGI DHWQVDYTHY EDKIILVWVE TNSGLIYAE RVKGETGQEF RVQTMKWYAM FAPKSLQSDN GPAFVAESTQ LLMKYLGIEH TTGIPWNPQS QALVERTHQT L KNTLEKLI PMFNAFESAL AGTLITLNIK RKGGLGTSPM DIFIFNKEQQ RIQQQSKSKQ EKIRFCYYRT RKRGHPGEWQ GP TQVLWGG DGAIVVKDRG TDRYLVIANK DVKFIPPPKE IQKE UniProtKB: Gag-Pol polyprotein |
-Macromolecule #2: vDNA, non-transfered strand
| Macromolecule | Name: vDNA, non-transfered strand / type: dna / ID: 2 / Number of copies: 2 / Classification: DNA |
|---|---|
| Source (natural) | Organism:  Visna lentivirus (strain 1514) Visna lentivirus (strain 1514) |
| Molecular weight | Theoretical: 6.456146 KDa |
| Sequence | String: (DG)(DC)(DT)(DG)(DC)(DG)(DA)(DG)(DA)(DT) (DC)(DC)(DG)(DC)(DT)(DC)(DC)(DG)(DG)(DT) (DG) |
-Macromolecule #3: vDNA-tDNA, transferred strand, joined to a model tDNA
| Macromolecule | Name: vDNA-tDNA, transferred strand, joined to a model tDNA / type: dna / ID: 3 / Number of copies: 2 / Classification: DNA |
|---|---|
| Source (natural) | Organism:  Visna lentivirus (strain 1514) Visna lentivirus (strain 1514) |
| Molecular weight | Theoretical: 15.387863 KDa |
| Sequence | String: (DA)(DA)(DC)(DA)(DC)(DC)(DG)(DG)(DA)(DG) (DC)(DG)(DG)(DA)(DT)(DC)(DT)(DC)(DG)(DC) (DA)(DG)(DT)(DC)(DG)(DA)(DC)(DC)(DA) (DC)(DC)(DC)(DT)(DA)(DA)(DT)(DC)(DA)(DA) (DG) (DT)(DT)(DT)(DT)(DT)(DT)(DG)(DG) (DG)(DG) |
-Macromolecule #4: tDNA
| Macromolecule | Name: tDNA / type: dna / ID: 4 / Number of copies: 2 / Classification: DNA |
|---|---|
| Source (natural) | Organism: synthetic construct (others) |
| Molecular weight | Theoretical: 7.0736 KDa |
| Sequence | String: (DC)(DC)(DC)(DC)(DA)(DA)(DA)(DA)(DA)(DA) (DC)(DT)(DT)(DG)(DA)(DT)(DT)(DA)(DG)(DG) (DG)(DT)(DG) |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Concentration | 0.3 mg/mL | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Buffer | pH: 6.5 Component:
| ||||||||||||
| Grid | Model: Ted Pella, lacey carbon grids coated with ultrathin carbon Material: COPPER / Mesh: 400 / Support film - Material: CARBON / Support film - topology: CONTINUOUS / Support film - Film thickness: 2 / Pretreatment - Type: GLOW DISCHARGE / Pretreatment - Time: 30 sec. | ||||||||||||
| Vitrification | Cryogen name: ETHANE / Chamber humidity: 100 % / Chamber temperature: 293 K / Instrument: FEI VITROBOT MARK IV Details: To lower salt concentration before plunge-freezing, the grids were blotted for 0.5 s, immediately hydrated with a 4-ul drop of 200 mM NaCl, 3 mM CaCl2 and 25 mM BisTris-HCl pH 6.5 and ...Details: To lower salt concentration before plunge-freezing, the grids were blotted for 0.5 s, immediately hydrated with a 4-ul drop of 200 mM NaCl, 3 mM CaCl2 and 25 mM BisTris-HCl pH 6.5 and blotted again for 2.5 s followed by plunging into liquid ethane.. |
- Electron microscopy
Electron microscopy
| Microscope | FEI POLARA 300 |
|---|---|
| Image recording | Film or detector model: GATAN K2 SUMMIT (4k x 4k) / Detector mode: COUNTING / Number grids imaged: 1 / Number real images: 722 / Average electron dose: 1.47 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD |
| Experimental equipment |  Model: Tecnai Polara / Image courtesy: FEI Company |
+ Image processing
Image processing
-Atomic model buiding 1
| Refinement | Space: REAL / Protocol: OTHER |
|---|---|
| Output model |  PDB-5m0r: |
 Movie
Movie Controller
Controller














 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)