[English] 日本語
 Yorodumi
Yorodumi- EMDB-41104: 36-mer Flagellar Switch Complex - FliF, FliG, FliM and FliN formi... -
+ Open data
Open data
- Basic information
Basic information
| Entry |  | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | 36-mer Flagellar Switch Complex - FliF, FliG, FliM and FliN forming C-ring from Salmonella | |||||||||
 Map data Map data | ||||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | Domain Swap / Symmetry mismatch / Flagellar component / Switch complex / C36 / 36-mer / MOTOR PROTEIN | |||||||||
| Biological species |  Salmonella enterica subsp. enterica serovar Typhimurium (bacteria) Salmonella enterica subsp. enterica serovar Typhimurium (bacteria) | |||||||||
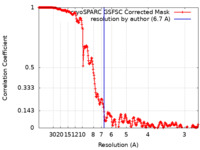
| Method | single particle reconstruction / cryo EM / Resolution: 6.7 Å | |||||||||
 Authors Authors | Singh PK / Iverson TM | |||||||||
| Funding support |  United States, 1 items United States, 1 items
| |||||||||
 Citation Citation |  Journal: Nat Microbiol / Year: 2024 Journal: Nat Microbiol / Year: 2024Title: CryoEM structures reveal how the bacterial flagellum rotates and switches direction. Authors: Prashant K Singh / Pankaj Sharma / Oshri Afanzar / Margo H Goldfarb / Elena Maklashina / Michael Eisenbach / Gary Cecchini / T M Iverson /   Abstract: Bacterial chemotaxis requires bidirectional flagellar rotation at different rates. Rotation is driven by a flagellar motor, which is a supercomplex containing multiple rings. Architectural ...Bacterial chemotaxis requires bidirectional flagellar rotation at different rates. Rotation is driven by a flagellar motor, which is a supercomplex containing multiple rings. Architectural uncertainty regarding the cytoplasmic C-ring, or 'switch', limits our understanding of how the motor transmits torque and direction to the flagellar rod. Here we report cryogenic electron microscopy structures for Salmonella enterica serovar typhimurium inner membrane MS-ring and C-ring in a counterclockwise pose (4.0 Å) and isolated C-ring in a clockwise pose alone (4.6 Å) and bound to a regulator (5.9 Å). Conformational differences between rotational poses include a 180° shift in FliF/FliG domains that rotates the outward-facing MotA/B binding site to inward facing. The regulator has specificity for the clockwise pose by bridging elements unique to this conformation. We used these structures to propose how the switch reverses rotation and transmits torque to the flagellum, which advances the understanding of bacterial chemotaxis and bidirectional motor rotation. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Supplemental images |
|---|
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_41104.map.gz emd_41104.map.gz | 845.1 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-41104-v30.xml emd-41104-v30.xml emd-41104.xml emd-41104.xml | 13.7 KB 13.7 KB | Display Display |  EMDB header EMDB header |
| FSC (resolution estimation) |  emd_41104_fsc.xml emd_41104_fsc.xml | 25.5 KB | Display |  FSC data file FSC data file |
| Images |  emd_41104.png emd_41104.png | 113.4 KB | ||
| Masks |  emd_41104_msk_1.map emd_41104_msk_1.map | 1.7 GB |  Mask map Mask map | |
| Filedesc metadata |  emd-41104.cif.gz emd-41104.cif.gz | 4 KB | ||
| Others |  emd_41104_half_map_1.map.gz emd_41104_half_map_1.map.gz emd_41104_half_map_2.map.gz emd_41104_half_map_2.map.gz | 1.6 GB 1.6 GB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-41104 http://ftp.pdbj.org/pub/emdb/structures/EMD-41104 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-41104 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-41104 | HTTPS FTP |
-Related structure data
| Related structure data |  8t8pC  8vibC  8vidC  8vkqC  8vkrC  41100 C: citing same article ( |
|---|
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|
- Map
Map
| File |  Download / File: emd_41104.map.gz / Format: CCP4 / Size: 1.7 GB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_41104.map.gz / Format: CCP4 / Size: 1.7 GB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||
| Voxel size |
| ||||||||||||||||||||||||||||||||||||
| Density |
| ||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
|
-Supplemental data
-Mask #1
| File |  emd_41104_msk_1.map emd_41104_msk_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Half map: #1
| File | emd_41104_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
| Density Histograms |
- Sample components
Sample components
-Entire : Flagellar MS-ring and C-ring complex containing FliF, FliG, FliM,...
| Entire | Name: Flagellar MS-ring and C-ring complex containing FliF, FliG, FliM, and FliN |
|---|---|
| Components |
|
-Supramolecule #1: Flagellar MS-ring and C-ring complex containing FliF, FliG, FliM,...
| Supramolecule | Name: Flagellar MS-ring and C-ring complex containing FliF, FliG, FliM, and FliN type: complex / ID: 1 / Parent: 0 |
|---|---|
| Source (natural) | Organism:  Salmonella enterica subsp. enterica serovar Typhimurium (bacteria) Salmonella enterica subsp. enterica serovar Typhimurium (bacteria) |
| Molecular weight | Theoretical: 3.5 MDa |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Buffer | pH: 7.5 |
|---|---|
| Vitrification | Cryogen name: ETHANE |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Image recording | Film or detector model: GATAN K3 (6k x 4k) / Average electron dose: 51.557 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD / Nominal defocus max: 2.0 µm / Nominal defocus min: 1.0 µm |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
 Movie
Movie Controller
Controller











 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)