+ Open data
Open data
- Basic information
Basic information
| Entry | Database: EMDB / ID: EMD-4101 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | Structure of F-ATPase from Pichia angusta, state2. | |||||||||
 Map data Map data | This is one of three states of the ATP synthase with the inhibitor protein bound. | |||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | ATP synthase / ATP hydrolase / complex / hydrolase | |||||||||
| Function / homology |  Function and homology information Function and homology informationnegative regulation of mitochondrial ATP synthesis coupled proton transport / angiostatin binding / negative regulation of hydrolase activity / ATPase inhibitor activity / heme biosynthetic process / negative regulation of endothelial cell proliferation / proton transmembrane transporter activity / proton motive force-driven ATP synthesis / proton-transporting two-sector ATPase complex, proton-transporting domain / proton motive force-driven mitochondrial ATP synthesis ...negative regulation of mitochondrial ATP synthesis coupled proton transport / angiostatin binding / negative regulation of hydrolase activity / ATPase inhibitor activity / heme biosynthetic process / negative regulation of endothelial cell proliferation / proton transmembrane transporter activity / proton motive force-driven ATP synthesis / proton-transporting two-sector ATPase complex, proton-transporting domain / proton motive force-driven mitochondrial ATP synthesis / H+-transporting two-sector ATPase / proton-transporting ATP synthase complex / proton-transporting ATP synthase activity, rotational mechanism / erythrocyte differentiation / ADP binding / ATPase binding / protein homotetramerization / calmodulin binding / mitochondrial inner membrane / lipid binding / structural molecule activity / cell surface / protein homodimerization activity / ATP hydrolysis activity / protein-containing complex / mitochondrion / ATP binding / identical protein binding / cytoplasm Similarity search - Function | |||||||||
| Biological species |  Ogataea angusta (fungus) / Ogataea angusta (fungus) /  | |||||||||
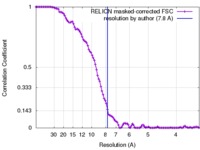
| Method | single particle reconstruction / cryo EM / Resolution: 7.8 Å | |||||||||
 Authors Authors | Vinothkumar KR / Montgomery MG | |||||||||
 Citation Citation |  Journal: Proc Natl Acad Sci U S A / Year: 2016 Journal: Proc Natl Acad Sci U S A / Year: 2016Title: Structure of the mitochondrial ATP synthase from determined by electron cryo-microscopy. Authors: Kutti R Vinothkumar / Martin G Montgomery / Sidong Liu / John E Walker /  Abstract: The structure of the intact monomeric ATP synthase from the fungus, , has been solved by electron cryo-microscopy. The structure provides insights into the mechanical coupling of the transmembrane ...The structure of the intact monomeric ATP synthase from the fungus, , has been solved by electron cryo-microscopy. The structure provides insights into the mechanical coupling of the transmembrane proton motive force across mitochondrial membranes in the synthesis of ATP. This mechanism requires a strong and integral stator, consisting of the catalytic αβ-domain, peripheral stalk, and, in the membrane domain, subunit a and associated supernumerary subunits, kept in contact with the rotor turning at speeds up to 350 Hz. The stator's integrity is ensured by robust attachment of both the oligomycin sensitivity conferral protein (OSCP) to the catalytic domain and the membrane domain of subunit b to subunit a. The ATP8 subunit provides an additional brace between the peripheral stalk and subunit a. At the junction between the OSCP and the apparently stiff, elongated α-helical b-subunit and associated d- and h-subunits, an elbow or joint allows the stator to bend to accommodate lateral movements during the activity of the catalytic domain. The stator may also apply lateral force to help keep the static a-subunit and rotating c-ring together. The interface between the c-ring and the a-subunit contains the transmembrane pathway for protons, and their passage across the membrane generates the turning of the rotor. The pathway has two half-channels containing conserved polar residues provided by a bundle of four α-helices inclined at ∼30° to the plane of the membrane, similar to those described in other species. The structure provides more insights into the workings of this amazing machine. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Movie |
 Movie viewer Movie viewer |
|---|---|
| Structure viewer | EM map:  SurfView SurfView Molmil Molmil Jmol/JSmol Jmol/JSmol |
| Supplemental images |
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_4101.map.gz emd_4101.map.gz | 91 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-4101-v30.xml emd-4101-v30.xml emd-4101.xml emd-4101.xml | 33.9 KB 33.9 KB | Display Display |  EMDB header EMDB header |
| FSC (resolution estimation) |  emd_4101_fsc.xml emd_4101_fsc.xml | 10.5 KB | Display |  FSC data file FSC data file |
| Images |  emd_4101.png emd_4101.png | 51.5 KB | ||
| Filedesc metadata |  emd-4101.cif.gz emd-4101.cif.gz | 8.8 KB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-4101 http://ftp.pdbj.org/pub/emdb/structures/EMD-4101 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-4101 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-4101 | HTTPS FTP |
-Related structure data
| Related structure data |  5lqyMC  4100C  4102C  5lqxC  5lqzC M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_4101.map.gz / Format: CCP4 / Size: 103 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_4101.map.gz / Format: CCP4 / Size: 103 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | This is one of three states of the ATP synthase with the inhibitor protein bound. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 1.75 Å | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Density |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
CCP4 map header:
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
-Supplemental data
- Sample components
Sample components
+Entire : Yeast F1FO ATP Synthase
+Supramolecule #1: Yeast F1FO ATP Synthase
+Supramolecule #2: Yeast F1FO ATP Synthase
+Supramolecule #3: ATP synthase inhibitor protein IF1
+Macromolecule #1: ATP synthase subunit f
+Macromolecule #2: ATP synthase subunit AAP1
+Macromolecule #3: ATP synthase subunit a
+Macromolecule #4: ATP synthase subunit b
+Macromolecule #5: ATP synthase alpha subunit
+Macromolecule #6: ATP synthase beta subunit
+Macromolecule #7: ATP synthase gamma subunit
+Macromolecule #8: ATP synthase delta subunit
+Macromolecule #9: ATP synthase epsilon subunit
+Macromolecule #10: ATP synthase inhibitor protein IF1
+Macromolecule #11: ATP synthase c subunit
+Macromolecule #12: ATP synthase OSCP subunit
+Macromolecule #13: ATP synthase subunit b
+Macromolecule #14: ATP synthase subunit d
+Macromolecule #15: ATP synthase subunit h
+Macromolecule #16: ATP synthase subunit a
+Macromolecule #17: ATP synthase subunit a
+Macromolecule #18: ADENOSINE-5'-TRIPHOSPHATE
+Macromolecule #19: MAGNESIUM ION
+Macromolecule #20: ADENOSINE-5'-DIPHOSPHATE
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Concentration | 3.5 mg/mL | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Buffer | pH: 7.5 Component:
| ||||||||||||
| Grid | Model: Quantifoil R0.6/1 / Material: GOLD / Mesh: 300 / Support film - Material: CARBON / Support film - topology: HOLEY / Pretreatment - Type: GLOW DISCHARGE / Pretreatment - Time: 75 sec. / Pretreatment - Atmosphere: AIR | ||||||||||||
| Vitrification | Cryogen name: ETHANE / Chamber humidity: 100 % / Chamber temperature: 277.15 K / Instrument: HOMEMADE PLUNGER Details: Grids were blotted for 12-14 seconds before plunging.. | ||||||||||||
| Details | The enzyme with bound inhibitor protein extracted in DDM and purified in Cymal-7. |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Temperature | Min: 87.5 K |
| Image recording | Film or detector model: FEI FALCON II (4k x 4k) / Detector mode: INTEGRATING / Digitization - Dimensions - Width: 4096 pixel / Digitization - Dimensions - Height: 4096 pixel / Digitization - Frames/image: 1-32 / Average exposure time: 4.0 sec. / Average electron dose: 64.0 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | C2 aperture diameter: 70.0 µm / Calibrated magnification: 81395 / Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD / Cs: 2.7 mm / Nominal defocus max: 5.0 µm / Nominal defocus min: 1.8 µm / Nominal magnification: 47000 |
| Sample stage | Specimen holder model: FEI TITAN KRIOS AUTOGRID HOLDER / Cooling holder cryogen: NITROGEN |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
+ Image processing
Image processing
-Atomic model buiding 1
| Details | The refinement of whole data was done at 1.72 A sampling. The map was scaled to 1.75 A after comparison with the model. |
|---|---|
| Refinement | Space: REAL / Protocol: RIGID BODY FIT |
| Output model |  PDB-5lqy: |
 Movie
Movie Controller
Controller















 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)