[English] 日本語
 Yorodumi
Yorodumi- EMDB-4037: Cryo-EM structure of the Anaphase-promoting complex/Cyclosome, in... -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: EMDB / ID: EMD-4037 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | Cryo-EM structure of the Anaphase-promoting complex/Cyclosome, in complex with the Mitotic checkpoint complex (APC/C-MCC) at 4.2 angstrom resolution | |||||||||
 Map data Map data | None | |||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | Complex / Ubiquitin / E3 ligase / Ubiquitin ligase / Cullin / RING / Cell cycle / Mitosis / Spindle checkpoint / Degron | |||||||||
| Function / homology |  Function and homology information Function and homology informationmitotic spindle assembly checkpoint MAD1-MAD2 complex / metaphase/anaphase transition of cell cycle / metaphase/anaphase transition of meiosis I / Inhibition of the proteolytic activity of APC/C required for the onset of anaphase by mitotic spindle checkpoint components / mitotic checkpoint complex / positive regulation of anaphase-promoting complex-dependent catabolic process / positive regulation of synapse maturation / regulation of meiotic nuclear division / Conversion from APC/C:Cdc20 to APC/C:Cdh1 in late anaphase / regulation of mitotic cell cycle spindle assembly checkpoint ...mitotic spindle assembly checkpoint MAD1-MAD2 complex / metaphase/anaphase transition of cell cycle / metaphase/anaphase transition of meiosis I / Inhibition of the proteolytic activity of APC/C required for the onset of anaphase by mitotic spindle checkpoint components / mitotic checkpoint complex / positive regulation of anaphase-promoting complex-dependent catabolic process / positive regulation of synapse maturation / regulation of meiotic nuclear division / Conversion from APC/C:Cdc20 to APC/C:Cdh1 in late anaphase / regulation of mitotic cell cycle spindle assembly checkpoint / establishment of centrosome localization / positive regulation of mitotic cell cycle spindle assembly checkpoint / meiotic sister chromatid cohesion, centromeric / Inactivation of APC/C via direct inhibition of the APC/C complex / APC/C:Cdc20 mediated degradation of mitotic proteins / regulation of dendrite development / positive regulation of synaptic plasticity / anaphase-promoting complex / Phosphorylation of Emi1 / regulation of meiotic cell cycle / Aberrant regulation of mitotic exit in cancer due to RB1 defects / anaphase-promoting complex-dependent catabolic process / protein branched polyubiquitination / metaphase/anaphase transition of mitotic cell cycle / Phosphorylation of the APC/C / anaphase-promoting complex binding / regulation of exit from mitosis / protein localization to chromosome, centromeric region / nuclear pore nuclear basket / outer kinetochore / positive regulation of mitotic metaphase/anaphase transition / positive regulation of ubiquitin protein ligase activity / ubiquitin ligase activator activity / positive regulation of dendrite morphogenesis / protein K11-linked ubiquitination / negative regulation of mitotic cell cycle / regulation of mitotic metaphase/anaphase transition / mitotic sister chromatid cohesion / ubiquitin-ubiquitin ligase activity / mitotic metaphase chromosome alignment / mitotic spindle assembly checkpoint signaling / negative regulation of ubiquitin protein ligase activity / Regulation of APC/C activators between G1/S and early anaphase / Transcriptional Regulation by VENTX / mitotic sister chromatid segregation / establishment of mitotic spindle orientation / cullin family protein binding / mitotic spindle assembly / enzyme-substrate adaptor activity / positive regulation of axon extension / protein K48-linked ubiquitination / ubiquitin-like ligase-substrate adaptor activity / intercellular bridge / heterochromatin / Amplification of signal from unattached kinetochores via a MAD2 inhibitory signal / nuclear periphery / Mitotic Prometaphase / APC/C:Cdc20 mediated degradation of Cyclin B / regulation of mitotic cell cycle / EML4 and NUDC in mitotic spindle formation / APC-Cdc20 mediated degradation of Nek2A / Resolution of Sister Chromatid Cohesion / Autodegradation of Cdh1 by Cdh1:APC/C / APC/C:Cdc20 mediated degradation of Securin / SCF-beta-TrCP mediated degradation of Emi1 / Assembly of the pre-replicative complex / Cdc20:Phospho-APC/C mediated degradation of Cyclin A / RHO GTPases Activate Formins / APC/C:Cdh1 mediated degradation of Cdc20 and other APC/C:Cdh1 targeted proteins in late mitosis/early G1 / negative regulation of protein catabolic process / G protein-coupled receptor binding / brain development / kinetochore / CDK-mediated phosphorylation and removal of Cdc6 / spindle / histone deacetylase binding / neuron projection development / spindle pole / ubiquitin-protein transferase activity / mitotic spindle / Separation of Sister Chromatids / ubiquitin protein ligase activity / nervous system development / mitotic cell cycle / Antigen processing: Ubiquitination & Proteasome degradation / microtubule cytoskeleton / Senescence-Associated Secretory Phenotype (SASP) / protein phosphatase binding / ubiquitin-dependent protein catabolic process / molecular adaptor activity / cell differentiation / protein kinase activity / non-specific serine/threonine protein kinase / Ub-specific processing proteases / ciliary basal body / protein ubiquitination / negative regulation of gene expression / cell division / protein serine kinase activity / protein serine/threonine kinase activity Similarity search - Function | |||||||||
| Biological species |  Homo sapiens (human) Homo sapiens (human) | |||||||||
| Method | single particle reconstruction / cryo EM / Resolution: 4.2 Å | |||||||||
 Authors Authors | Alfieri C / Chang L / Zhang Z / Yang J / Maslen S / Skehel M / Barford D | |||||||||
 Citation Citation |  Journal: Nature / Year: 2016 Journal: Nature / Year: 2016Title: Molecular basis of APC/C regulation by the spindle assembly checkpoint. Authors: Claudio Alfieri / Leifu Chang / Ziguo Zhang / Jing Yang / Sarah Maslen / Mark Skehel / David Barford /  Abstract: In the dividing eukaryotic cell, the spindle assembly checkpoint (SAC) ensures that each daughter cell inherits an identical set of chromosomes. The SAC coordinates the correct attachment of sister ...In the dividing eukaryotic cell, the spindle assembly checkpoint (SAC) ensures that each daughter cell inherits an identical set of chromosomes. The SAC coordinates the correct attachment of sister chromatid kinetochores to the mitotic spindle with activation of the anaphase-promoting complex (APC/C), the E3 ubiquitin ligase responsible for initiating chromosome separation. In response to unattached kinetochores, the SAC generates the mitotic checkpoint complex (MCC), which inhibits the APC/C and delays chromosome segregation. By cryo-electron microscopy, here we determine the near-atomic resolution structure of a human APC/C–MCC complex (APC/C(MCC)). Degron-like sequences of the MCC subunit BubR1 block degron recognition sites on Cdc20, the APC/C coactivator subunit responsible for substrate interactions. BubR1 also obstructs binding of the initiating E2 enzyme UbcH10 to repress APC/C ubiquitination activity. Conformational variability of the complex enables UbcH10 association, and structural analysis shows how the Cdc20 subunit intrinsic to the MCC (Cdc20(MCC)) is ubiquitinated, a process that results in APC/C reactivation when the SAC is silenced. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Movie |
 Movie viewer Movie viewer |
|---|---|
| Structure viewer | EM map:  SurfView SurfView Molmil Molmil Jmol/JSmol Jmol/JSmol |
| Supplemental images |
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_4037.map.gz emd_4037.map.gz | 8.5 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-4037-v30.xml emd-4037-v30.xml emd-4037.xml emd-4037.xml | 42.7 KB 42.7 KB | Display Display |  EMDB header EMDB header |
| Images |  emd_4037_1.png emd_4037_1.png emd_4037_2.png emd_4037_2.png emd_4037_3.png emd_4037_3.png emd_4037_4.png emd_4037_4.png | 76 KB 196.4 KB 185.1 KB 216.3 KB | ||
| Filedesc metadata |  emd-4037.cif.gz emd-4037.cif.gz | 11.6 KB | ||
| Others |  emd_4037_additional_1.map.gz emd_4037_additional_1.map.gz emd_4037_additional_2.map.gz emd_4037_additional_2.map.gz emd_4037_additional_3.map.gz emd_4037_additional_3.map.gz | 33.5 MB 7.8 MB 7.4 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-4037 http://ftp.pdbj.org/pub/emdb/structures/EMD-4037 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-4037 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-4037 | HTTPS FTP |
-Related structure data
| Related structure data |  5lcwMC M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_4037.map.gz / Format: CCP4 / Size: 70.2 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_4037.map.gz / Format: CCP4 / Size: 70.2 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | None | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 1.36 Å | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Density |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
CCP4 map header:
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
-Supplemental data
-Additional map: None
| File | emd_4037_additional_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | None | ||||||||||||
| Projections & Slices |
| ||||||||||||
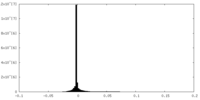
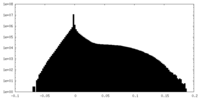
| Density Histograms |
-Additional map: None
| File | emd_4037_additional_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | None | ||||||||||||
| Projections & Slices |
| ||||||||||||
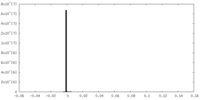
| Density Histograms |
-Additional map: None
| File | emd_4037_additional_3.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | None | ||||||||||||
| Projections & Slices |
| ||||||||||||
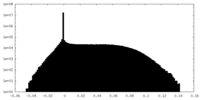
| Density Histograms |
- Sample components
Sample components
+Entire : Anaphase-promoting complex/Cyclosome, in complex with the Mitotic...
+Supramolecule #1: Anaphase-promoting complex/Cyclosome, in complex with the Mitotic...
+Macromolecule #1: Anaphase-promoting complex subunit 1
+Macromolecule #2: Anaphase-promoting complex subunit 11
+Macromolecule #3: Cell division cycle protein 23 homolog
+Macromolecule #4: Anaphase-promoting complex subunit 15
+Macromolecule #5: Anaphase-promoting complex subunit 16
+Macromolecule #6: Cell division cycle protein 27 homolog
+Macromolecule #7: Anaphase-promoting complex subunit CDC26
+Macromolecule #8: Anaphase-promoting complex subunit 4
+Macromolecule #9: Cell division cycle protein 16 homolog
+Macromolecule #10: Anaphase-promoting complex subunit 10
+Macromolecule #11: Anaphase-promoting complex subunit 13
+Macromolecule #12: Anaphase-promoting complex subunit 2
+Macromolecule #13: Anaphase-promoting complex subunit 5
+Macromolecule #14: Cell division cycle protein 20 homolog
+Macromolecule #15: Cell division cycle protein 20 homolog
+Macromolecule #16: Mitotic checkpoint serine/threonine-protein kinase BUB1 beta,Mito...
+Macromolecule #17: Anaphase-promoting complex subunit 7
+Macromolecule #18: Mitotic spindle assembly checkpoint protein MAD2A
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Buffer | pH: 8 |
|---|---|
| Vitrification | Cryogen name: ETHANE |
- Electron microscopy
Electron microscopy
| Microscope | FEI POLARA 300 |
|---|---|
| Image recording | Film or detector model: FEI FALCON II (4k x 4k) / Average electron dose: 27.0 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD |
| Experimental equipment |  Model: Tecnai Polara / Image courtesy: FEI Company |
- Image processing
Image processing
| Startup model | Type of model: EMDB MAP EMDB ID: |
|---|---|
| Final reconstruction | Resolution.type: BY AUTHOR / Resolution: 4.2 Å / Resolution method: FSC 0.143 CUT-OFF / Software - Name: RELION (ver. 1.4) / Number images used: 155263 |
| Initial angle assignment | Type: NOT APPLICABLE |
| Final angle assignment | Type: NOT APPLICABLE |
 Movie
Movie Controller
Controller

































 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)








































 Trichoplusia ni (cabbage looper)
Trichoplusia ni (cabbage looper)
