[English] 日本語
 Yorodumi
Yorodumi- EMDB-39161: CryoEM structure of M. tuberculosis ClpXP1P2 complex bound to bor... -
+ Open data
Open data
- Basic information
Basic information
| Entry |  | ||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Title | CryoEM structure of M. tuberculosis ClpXP1P2 complex bound to bortezomib | ||||||||||||||||||||||||||||||
 Map data Map data | |||||||||||||||||||||||||||||||
 Sample Sample |
| ||||||||||||||||||||||||||||||
 Keywords Keywords | Mycobacterium tuberculosis / Caseinolytic protease system / Activation / HYDROLASE | ||||||||||||||||||||||||||||||
| Function / homology |  Function and homology information Function and homology informationnegative regulation of protein polymerization / HslUV protease complex / endopeptidase Clp / endopeptidase Clp complex / ATP-dependent peptidase activity / protein quality control for misfolded or incompletely synthesized proteins / peptidoglycan-based cell wall / : / ATP-dependent protein folding chaperone / unfolded protein binding ...negative regulation of protein polymerization / HslUV protease complex / endopeptidase Clp / endopeptidase Clp complex / ATP-dependent peptidase activity / protein quality control for misfolded or incompletely synthesized proteins / peptidoglycan-based cell wall / : / ATP-dependent protein folding chaperone / unfolded protein binding / ATPase binding / protein dimerization activity / serine-type endopeptidase activity / cell division / ATP hydrolysis activity / zinc ion binding / ATP binding / plasma membrane / cytosol Similarity search - Function | ||||||||||||||||||||||||||||||
| Biological species |   Mycobacterium tuberculosis H37Rv (bacteria) Mycobacterium tuberculosis H37Rv (bacteria) | ||||||||||||||||||||||||||||||
| Method | single particle reconstruction / cryo EM / Resolution: 2.24 Å | ||||||||||||||||||||||||||||||
 Authors Authors | Zhou B / Zhao H / Gao Y / Chen X / Zhang T / He J / Xiong X | ||||||||||||||||||||||||||||||
| Funding support |  China, 9 items China, 9 items
| ||||||||||||||||||||||||||||||
 Citation Citation |  Journal: To Be Published Journal: To Be PublishedTitle: Activation mechanism of caseinolytic chaperone-protease system in Mycobacterium tuberculosis by the anti-cancer drug bortezomib Authors: Zhou B / Zhao H / Gao Y / Chen X / Zhang T / He J / Xiong X | ||||||||||||||||||||||||||||||
| History |
|
- Structure visualization
Structure visualization
| Supplemental images |
|---|
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_39161.map.gz emd_39161.map.gz | 243.8 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-39161-v30.xml emd-39161-v30.xml emd-39161.xml emd-39161.xml | 20.7 KB 20.7 KB | Display Display |  EMDB header EMDB header |
| FSC (resolution estimation) |  emd_39161_fsc.xml emd_39161_fsc.xml | 23.3 KB | Display |  FSC data file FSC data file |
| Images |  emd_39161.png emd_39161.png | 41.1 KB | ||
| Masks |  emd_39161_msk_1.map emd_39161_msk_1.map | 512 MB |  Mask map Mask map | |
| Filedesc metadata |  emd-39161.cif.gz emd-39161.cif.gz | 6.3 KB | ||
| Others |  emd_39161_half_map_1.map.gz emd_39161_half_map_1.map.gz emd_39161_half_map_2.map.gz emd_39161_half_map_2.map.gz | 471.5 MB 471.5 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-39161 http://ftp.pdbj.org/pub/emdb/structures/EMD-39161 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-39161 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-39161 | HTTPS FTP |
-Validation report
| Summary document |  emd_39161_validation.pdf.gz emd_39161_validation.pdf.gz | 1.1 MB | Display |  EMDB validaton report EMDB validaton report |
|---|---|---|---|---|
| Full document |  emd_39161_full_validation.pdf.gz emd_39161_full_validation.pdf.gz | 1.1 MB | Display | |
| Data in XML |  emd_39161_validation.xml.gz emd_39161_validation.xml.gz | 25.9 KB | Display | |
| Data in CIF |  emd_39161_validation.cif.gz emd_39161_validation.cif.gz | 34.2 KB | Display | |
| Arichive directory |  https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-39161 https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-39161 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-39161 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-39161 | HTTPS FTP |
-Related structure data
| Related structure data |  8yd0MC  8ycxC  8yd1C  8yd2C  8yd4C  9jvpC  9jvzC M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_39161.map.gz / Format: CCP4 / Size: 512 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_39161.map.gz / Format: CCP4 / Size: 512 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 0.71 Å | ||||||||||||||||||||||||||||||||||||
| Density |
| ||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
|
-Supplemental data
-Mask #1
| File |  emd_39161_msk_1.map emd_39161_msk_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Half map: #2
| File | emd_39161_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Half map: #1
| File | emd_39161_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
| Density Histograms |
- Sample components
Sample components
-Entire : CryoEM structure of M. tuberculosis ClpXP1P2 complex bound to bor...
| Entire | Name: CryoEM structure of M. tuberculosis ClpXP1P2 complex bound to bortezomib |
|---|---|
| Components |
|
-Supramolecule #1: CryoEM structure of M. tuberculosis ClpXP1P2 complex bound to bor...
| Supramolecule | Name: CryoEM structure of M. tuberculosis ClpXP1P2 complex bound to bortezomib type: complex / ID: 1 / Parent: 0 / Macromolecule list: #1-#3 |
|---|---|
| Source (natural) | Organism:  |
-Macromolecule #1: ATP-dependent Clp protease ATP-binding subunit ClpX
| Macromolecule | Name: ATP-dependent Clp protease ATP-binding subunit ClpX / type: protein_or_peptide / ID: 1 / Number of copies: 6 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Mycobacterium tuberculosis H37Rv (bacteria) / Strain: H37Rv Mycobacterium tuberculosis H37Rv (bacteria) / Strain: H37Rv |
| Molecular weight | Theoretical: 39.05775 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: ELPKPAEIRE FLEGYVIGQD TAKRTLAVAV YNHYKRIQAG EKGRDSRCEP VELTKSNILM LGPTGCGKTY LAQTLAKMLN VPFAIADAT ALTEAGYVGE DVENILLKLI QAADYDVKRA ETGIIYIDQV DKIARKSENP SITRDVSGEG VQQALLKILE G TQASVPPQ ...String: ELPKPAEIRE FLEGYVIGQD TAKRTLAVAV YNHYKRIQAG EKGRDSRCEP VELTKSNILM LGPTGCGKTY LAQTLAKMLN VPFAIADAT ALTEAGYVGE DVENILLKLI QAADYDVKRA ETGIIYIDQV DKIARKSENP SITRDVSGEG VQQALLKILE G TQASVPPQ GGRKHPHQEF IQIDTTNVLF IVAGAFAGLE KIIYERVGKR GLGFGAEVRS KAEIDTTDHF ADVMPEDLIK FG LIPEFIG RLPVVASVTN LDKESLVKIL SEPKNALVKQ YIRLFEMDGV ELEFTDDALE AIADQAIHRG TGARGLRAIM EEV LLPVMY DIPSRDDVAK VVVTKETVQD NVLPTIVPR UniProtKB: ATP-dependent Clp protease ATP-binding subunit ClpX |
-Macromolecule #2: ATP-dependent Clp protease proteolytic subunit 2
| Macromolecule | Name: ATP-dependent Clp protease proteolytic subunit 2 / type: protein_or_peptide / ID: 2 / Number of copies: 7 / Enantiomer: LEVO / EC number: endopeptidase Clp |
|---|---|
| Source (natural) | Organism:  Mycobacterium tuberculosis H37Rv (bacteria) / Strain: H37Rv Mycobacterium tuberculosis H37Rv (bacteria) / Strain: H37Rv |
| Molecular weight | Theoretical: 21.543566 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: ILPSFIEHSS FGVKESNPYN KLFEERIIFL GVQVDDASAN DIMAQLLVLE SLDPDRDITM YINSPGGGFT SLMAIYDTMQ YVRADIQTV CLGQAASAAA VLLAAGTPGK RMALPNARVL IHQPSLSGVI QGQFSDLEIQ AAEIERMRTL METTLARHTG K DAGVIRKD ...String: ILPSFIEHSS FGVKESNPYN KLFEERIIFL GVQVDDASAN DIMAQLLVLE SLDPDRDITM YINSPGGGFT SLMAIYDTMQ YVRADIQTV CLGQAASAAA VLLAAGTPGK RMALPNARVL IHQPSLSGVI QGQFSDLEIQ AAEIERMRTL METTLARHTG K DAGVIRKD TDRDKILTAE EAKDYGIIDT VLEYRKLS UniProtKB: ATP-dependent Clp protease proteolytic subunit 2 |
-Macromolecule #3: ATP-dependent Clp protease proteolytic subunit 1
| Macromolecule | Name: ATP-dependent Clp protease proteolytic subunit 1 / type: protein_or_peptide / ID: 3 / Number of copies: 7 / Enantiomer: LEVO / EC number: endopeptidase Clp |
|---|---|
| Source (natural) | Organism:  Mycobacterium tuberculosis H37Rv (bacteria) / Strain: H37Rv Mycobacterium tuberculosis H37Rv (bacteria) / Strain: H37Rv |
| Molecular weight | Theoretical: 19.383111 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: SLTDSVYERL LSERIIFLGS EVNDEIANRL CAQILLLAAE DASKDISLYI NSPGGSISAG MAIYDTMVLA PCDIATYAMG MAASMGEFL LAAGTKGKRY ALPHARILMH QPLGGVTGSA ADIAIQAEQF AVIKKEMFRL NAEFTGQPIE RIEADSDRDR W FTAAEALE YGFVDHIITR UniProtKB: ATP-dependent Clp protease proteolytic subunit 1 |
-Macromolecule #4: MAGNESIUM ION
| Macromolecule | Name: MAGNESIUM ION / type: ligand / ID: 4 / Number of copies: 2 / Formula: MG |
|---|---|
| Molecular weight | Theoretical: 24.305 Da |
-Macromolecule #5: ADENOSINE-5'-TRIPHOSPHATE
| Macromolecule | Name: ADENOSINE-5'-TRIPHOSPHATE / type: ligand / ID: 5 / Number of copies: 4 / Formula: ATP |
|---|---|
| Molecular weight | Theoretical: 507.181 Da |
| Chemical component information |  ChemComp-ATP: |
-Macromolecule #6: ADENOSINE-5'-DIPHOSPHATE
| Macromolecule | Name: ADENOSINE-5'-DIPHOSPHATE / type: ligand / ID: 6 / Number of copies: 2 / Formula: ADP |
|---|---|
| Molecular weight | Theoretical: 427.201 Da |
| Chemical component information |  ChemComp-ADP: |
-Macromolecule #7: N-[(1R)-1-(DIHYDROXYBORYL)-3-METHYLBUTYL]-N-(PYRAZIN-2-YLCARBONYL...
| Macromolecule | Name: N-[(1R)-1-(DIHYDROXYBORYL)-3-METHYLBUTYL]-N-(PYRAZIN-2-YLCARBONYL)-L-PHENYLALANINAMIDE type: ligand / ID: 7 / Number of copies: 14 / Formula: BO2 |
|---|---|
| Molecular weight | Theoretical: 384.237 Da |
| Chemical component information | 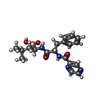 ChemComp-BO2: |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Buffer | pH: 7.5 |
|---|---|
| Vitrification | Cryogen name: ETHANE |
- Electron microscopy
Electron microscopy
| Microscope | TFS KRIOS |
|---|---|
| Image recording | Film or detector model: FEI FALCON IV (4k x 4k) / Average electron dose: 50.0 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD / Nominal defocus max: 2.4 µm / Nominal defocus min: 0.8 µm |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
 Movie
Movie Controller
Controller











 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)














































