+ Open data
Open data
- Basic information
Basic information
| Entry |  | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Title | Cryo-EM structure of OSCA1.2-DOPC-1:50-contracted state | ||||||||||||
 Map data Map data | |||||||||||||
 Sample Sample |
| ||||||||||||
 Keywords Keywords | OSCA/TMEM63 channel / mechanosensitive channel / PLANT PROTEIN | ||||||||||||
| Function / homology |  Function and homology information Function and homology informationmechanosensitive monoatomic ion channel activity / calcium-activated cation channel activity / monoatomic cation transport / identical protein binding / plasma membrane Similarity search - Function | ||||||||||||
| Biological species |  | ||||||||||||
| Method | single particle reconstruction / cryo EM / Resolution: 3.59 Å | ||||||||||||
 Authors Authors | Zhang Y / Han Y | ||||||||||||
| Funding support |  China, China,  Australia, 3 items Australia, 3 items
| ||||||||||||
 Citation Citation |  Journal: Nature / Year: 2024 Journal: Nature / Year: 2024Title: Mechanical activation opens a lipid-lined pore in OSCA ion channels. Authors: Yaoyao Han / Zijing Zhou / Ruitao Jin / Fei Dai / Yifan Ge / Xisan Ju / Xiaonuo Ma / Sitong He / Ling Yuan / Yingying Wang / Wei Yang / Xiaomin Yue / Zhongwen Chen / Yadong Sun / Ben Corry / ...Authors: Yaoyao Han / Zijing Zhou / Ruitao Jin / Fei Dai / Yifan Ge / Xisan Ju / Xiaonuo Ma / Sitong He / Ling Yuan / Yingying Wang / Wei Yang / Xiaomin Yue / Zhongwen Chen / Yadong Sun / Ben Corry / Charles D Cox / Yixiao Zhang /   Abstract: OSCA/TMEM63 channels are the largest known family of mechanosensitive channels, playing critical roles in plant and mammalian mechanotransduction. Here we determined 44 cryogenic electron microscopy ...OSCA/TMEM63 channels are the largest known family of mechanosensitive channels, playing critical roles in plant and mammalian mechanotransduction. Here we determined 44 cryogenic electron microscopy structures of OSCA/TMEM63 channels in different environments to investigate the molecular basis of OSCA/TMEM63 channel mechanosensitivity. In nanodiscs, we mimicked increased membrane tension and observed a dilated pore with membrane access in one of the OSCA1.2 subunits. In liposomes, we captured the fully open structure of OSCA1.2 in the inside-in orientation, in which the pore shows a large lateral opening to the membrane. Unusually for ion channels, structural, functional and computational evidence supports the existence of a 'proteo-lipidic pore' in which lipids act as a wall of the ion permeation pathway. In the less tension-sensitive homologue OSCA3.1, we identified an 'interlocking' lipid tightly bound in the central cleft, keeping the channel closed. Mutation of the lipid-coordinating residues induced OSCA3.1 activation, revealing a conserved open conformation of OSCA channels. Our structures provide a global picture of the OSCA channel gating cycle, uncover the importance of bound lipids and show that each subunit can open independently. This expands both our understanding of channel-mediated mechanotransduction and channel pore formation, with important mechanistic implications for the TMEM16 and TMC protein families. | ||||||||||||
| History |
|
- Structure visualization
Structure visualization
| Supplemental images |
|---|
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_38728.map.gz emd_38728.map.gz | 43 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-38728-v30.xml emd-38728-v30.xml emd-38728.xml emd-38728.xml | 18.1 KB 18.1 KB | Display Display |  EMDB header EMDB header |
| Images |  emd_38728.png emd_38728.png | 96.8 KB | ||
| Filedesc metadata |  emd-38728.cif.gz emd-38728.cif.gz | 6.2 KB | ||
| Others |  emd_38728_half_map_1.map.gz emd_38728_half_map_1.map.gz emd_38728_half_map_2.map.gz emd_38728_half_map_2.map.gz | 42.3 MB 42.3 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-38728 http://ftp.pdbj.org/pub/emdb/structures/EMD-38728 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-38728 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-38728 | HTTPS FTP |
-Validation report
| Summary document |  emd_38728_validation.pdf.gz emd_38728_validation.pdf.gz | 782.4 KB | Display |  EMDB validaton report EMDB validaton report |
|---|---|---|---|---|
| Full document |  emd_38728_full_validation.pdf.gz emd_38728_full_validation.pdf.gz | 782 KB | Display | |
| Data in XML |  emd_38728_validation.xml.gz emd_38728_validation.xml.gz | 11.9 KB | Display | |
| Data in CIF |  emd_38728_validation.cif.gz emd_38728_validation.cif.gz | 13.9 KB | Display | |
| Arichive directory |  https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-38728 https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-38728 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-38728 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-38728 | HTTPS FTP |
-Related structure data
| Related structure data |  8xw2MC  8xajC  8xngC  8xryC  8xs0C  8xs4C  8xs5C  8xvxC  8xvyC  8xvzC  8xw0C  8xw1C  8xw3C  8xw4C C: citing same article ( M: atomic model generated by this map |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|
- Map
Map
| File |  Download / File: emd_38728.map.gz / Format: CCP4 / Size: 52.7 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_38728.map.gz / Format: CCP4 / Size: 52.7 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 1.055 Å | ||||||||||||||||||||||||||||||||||||
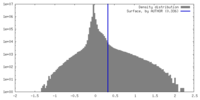
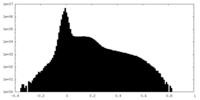
| Density |
| ||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
|
-Supplemental data
-Half map: #1
| File | emd_38728_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
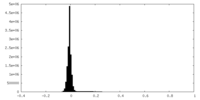
| Density Histograms |
-Half map: #2
| File | emd_38728_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
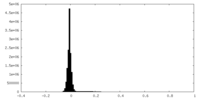
| Density Histograms |
- Sample components
Sample components
-Entire : The cryo-EM structure of OSCA1.2-DOPC-1:50-contracted state
| Entire | Name: The cryo-EM structure of OSCA1.2-DOPC-1:50-contracted state |
|---|---|
| Components |
|
-Supramolecule #1: The cryo-EM structure of OSCA1.2-DOPC-1:50-contracted state
| Supramolecule | Name: The cryo-EM structure of OSCA1.2-DOPC-1:50-contracted state type: complex / ID: 1 / Parent: 0 / Macromolecule list: all |
|---|---|
| Source (natural) | Organism:  |
-Macromolecule #1: Calcium permeable stress-gated cation channel 1
| Macromolecule | Name: Calcium permeable stress-gated cation channel 1 / type: protein_or_peptide / ID: 1 / Number of copies: 2 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  |
| Molecular weight | Theoretical: 88.615211 KDa |
| Recombinant expression | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Sequence | String: MATLQDIGVS AGINILSAFV FFIIFAVLRL QPFNDRVYFS KWYLKGLRSS PARGGAFAQR FVNLDFRSYM KFLNWMPEAL KMPEPELID HAGLDSVVYL RIYWLGLKIF TPIAVLAWAV LVPVNWTNNT LEMAKQLRNV TSSDIDKLSV SNIPEYSMRF W THIVMAYA ...String: MATLQDIGVS AGINILSAFV FFIIFAVLRL QPFNDRVYFS KWYLKGLRSS PARGGAFAQR FVNLDFRSYM KFLNWMPEAL KMPEPELID HAGLDSVVYL RIYWLGLKIF TPIAVLAWAV LVPVNWTNNT LEMAKQLRNV TSSDIDKLSV SNIPEYSMRF W THIVMAYA FTIWTCYVLM KEYETIANMR LQFVASEARR PDQFTVLVRN VPPDADESVS ELVEHFFLVN HPDHYLTHQV VC NANKLAD LVKKKKKLQN WLDYYQLKYA RNNSQRIMVK LGFLGLWGQK VDAIEHYIAE IDKISKEISK EREEVVNDPK AIM PAAFVS FKTRWAAAVC AQTQQTRNPT QWLTEWAPEP RDVFWSNLAI PYVSLTVRRL IMHVAFFFLT FFFIVPIAFV QSLA TIEGI VKAAPFLKFI VDDKFMKSVI QGFLPGIALK LFLAFLPSIL MIMSKFEGFT SISSLERRAA FRYYIFNLVN VFLAS VIAG AAFEQLNSFL NQSANQIPKT IGVAIPMKAT FFITYIMVDG WAGVAGEILM LKPLIMFHLK NAFLVKTDKD REEAMD PGS IGFNTGEPRI QLYFLLGLVY APVTPMLLPF ILVFFALAYI VYRHQIINVY NQEYESAAAF WPDVHGRVIA ALVISQL LL MGLLGTKHAA LAAPFLIALP VLTIGFHHFC KGRYEPAFIR YPLQEAMMKD TLETAREPNL NLKGYLQNAY VHPVFKGD E DDYDIDDKLG KFEDEAIIVP TKRQSRRNTP APSIISGDDS PSLPFSGKLV SNSLEV UniProtKB: Hyperosmolality-gated Ca2+ permeable channel 1.2 |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Buffer | pH: 7.9 |
|---|---|
| Vitrification | Cryogen name: ETHANE |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Image recording | Film or detector model: GATAN K3 (6k x 4k) / Average electron dose: 49.41 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD / Nominal defocus max: 2.4 µm / Nominal defocus min: 1.4000000000000001 µm |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
 Movie
Movie Controller
Controller



















 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)