+ Open data
Open data
- Basic information
Basic information
| Entry | Database: EMDB / ID: EMD-3838 | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Title | Cryo-EM structure of F-actin in complex with AppNHp (AMPPNP) | |||||||||||||||
 Map data Map data | Cryo-EM structure of F-actin in complex with AppNHp (AMPPNP) | |||||||||||||||
 Sample Sample |
| |||||||||||||||
 Keywords Keywords | Cytoskeleton / nucleotide states / filament stability / cell migration / STRUCTURAL PROTEIN | |||||||||||||||
| Function / homology |  Function and homology information Function and homology informationcytoskeletal motor activator activity / myosin heavy chain binding / tropomyosin binding / actin filament bundle / troponin I binding / filamentous actin / mesenchyme migration / skeletal muscle myofibril / actin filament bundle assembly / striated muscle thin filament ...cytoskeletal motor activator activity / myosin heavy chain binding / tropomyosin binding / actin filament bundle / troponin I binding / filamentous actin / mesenchyme migration / skeletal muscle myofibril / actin filament bundle assembly / striated muscle thin filament / skeletal muscle thin filament assembly / actin monomer binding / skeletal muscle fiber development / stress fiber / titin binding / actin filament polymerization / filopodium / actin filament / Hydrolases; Acting on acid anhydrides; Acting on acid anhydrides to facilitate cellular and subcellular movement / calcium-dependent protein binding / lamellipodium / cell body / hydrolase activity / protein domain specific binding / calcium ion binding / positive regulation of gene expression / magnesium ion binding / ATP binding / identical protein binding / cytoplasm Similarity search - Function | |||||||||||||||
| Biological species |  | |||||||||||||||
| Method | single particle reconstruction / cryo EM / Resolution: 3.6 Å | |||||||||||||||
 Authors Authors | Merino F / Pospich S | |||||||||||||||
| Funding support |  Germany, 4 items Germany, 4 items
| |||||||||||||||
 Citation Citation |  Journal: Nat Struct Mol Biol / Year: 2018 Journal: Nat Struct Mol Biol / Year: 2018Title: Structural transitions of F-actin upon ATP hydrolysis at near-atomic resolution revealed by cryo-EM. Authors: Felipe Merino / Sabrina Pospich / Johanna Funk / Thorsten Wagner / Florian Küllmer / Hans-Dieter Arndt / Peter Bieling / Stefan Raunser /  Abstract: The function of actin is coupled to the nucleotide bound to its active site. ATP hydrolysis is activated during polymerization; a delay between hydrolysis and inorganic phosphate (P) release results ...The function of actin is coupled to the nucleotide bound to its active site. ATP hydrolysis is activated during polymerization; a delay between hydrolysis and inorganic phosphate (P) release results in a gradient of ATP, ADP-P and ADP along actin filaments (F-actin). Actin-binding proteins can recognize F-actin's nucleotide state, using it as a local 'age' tag. The underlying mechanism is complex and poorly understood. Here we report six high-resolution cryo-EM structures of F-actin from rabbit skeletal muscle in different nucleotide states. The structures reveal that actin polymerization repositions the proposed catalytic base, His161, closer to the γ-phosphate. Nucleotide hydrolysis and P release modulate the conformational ensemble at the periphery of the filament, thus resulting in open and closed states, which can be sensed by coronin-1B. The drug-like toxin jasplakinolide locks F-actin in an open state. Our results demonstrate in detail how ATP hydrolysis links to F-actin's conformational dynamics and protein interaction. | |||||||||||||||
| History |
|
- Structure visualization
Structure visualization
| Movie |
 Movie viewer Movie viewer |
|---|---|
| Structure viewer | EM map:  SurfView SurfView Molmil Molmil Jmol/JSmol Jmol/JSmol |
| Supplemental images |
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_3838.map.gz emd_3838.map.gz | 62.4 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-3838-v30.xml emd-3838-v30.xml emd-3838.xml emd-3838.xml | 23.6 KB 23.6 KB | Display Display |  EMDB header EMDB header |
| Images |  emd_3838.png emd_3838.png | 128.6 KB | ||
| Masks |  emd_3838_msk_1.map emd_3838_msk_1.map | 67 MB |  Mask map Mask map | |
| Filedesc metadata |  emd-3838.cif.gz emd-3838.cif.gz | 6.9 KB | ||
| Others |  emd_3838_half_map_1.map.gz emd_3838_half_map_1.map.gz emd_3838_half_map_2.map.gz emd_3838_half_map_2.map.gz | 51.6 MB 51.6 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-3838 http://ftp.pdbj.org/pub/emdb/structures/EMD-3838 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-3838 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-3838 | HTTPS FTP |
-Validation report
| Summary document |  emd_3838_validation.pdf.gz emd_3838_validation.pdf.gz | 840.6 KB | Display |  EMDB validaton report EMDB validaton report |
|---|---|---|---|---|
| Full document |  emd_3838_full_validation.pdf.gz emd_3838_full_validation.pdf.gz | 839.9 KB | Display | |
| Data in XML |  emd_3838_validation.xml.gz emd_3838_validation.xml.gz | 12.3 KB | Display | |
| Data in CIF |  emd_3838_validation.cif.gz emd_3838_validation.cif.gz | 14.5 KB | Display | |
| Arichive directory |  https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-3838 https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-3838 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-3838 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-3838 | HTTPS FTP |
-Related structure data
| Related structure data |  5ooeMC  3835C  3836C  3837C  3839C  4259C  5onvC  5oocC  5oodC  5oofC  6fhlC C: citing same article ( M: atomic model generated by this map |
|---|---|
| Similar structure data |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_3838.map.gz / Format: CCP4 / Size: 67 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_3838.map.gz / Format: CCP4 / Size: 67 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Cryo-EM structure of F-actin in complex with AppNHp (AMPPNP) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 1.14 Å | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
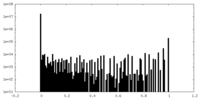
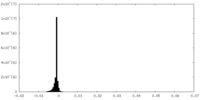
| Density |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
CCP4 map header:
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
-Supplemental data
-Mask #1
| File |  emd_3838_msk_1.map emd_3838_msk_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Half map: Cryo-EM structure of F-actin in complex with AppNHp...
| File | emd_3838_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Cryo-EM structure of F-actin in complex with AppNHp (AMPPNP) - First half volume | ||||||||||||
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Half map: Cryo-EM structure of F-actin in complex with AppNHp...
| File | emd_3838_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Cryo-EM structure of F-actin in complex with AppNHp (AMPPNP) - Second half volume | ||||||||||||
| Projections & Slices |
| ||||||||||||
| Density Histograms |
- Sample components
Sample components
-Entire : Filamentous alpha actin in complex with AppNHp (AMPPNP)
| Entire | Name: Filamentous alpha actin in complex with AppNHp (AMPPNP) |
|---|---|
| Components |
|
-Supramolecule #1: Filamentous alpha actin in complex with AppNHp (AMPPNP)
| Supramolecule | Name: Filamentous alpha actin in complex with AppNHp (AMPPNP) type: complex / ID: 1 / Parent: 0 / Macromolecule list: #1 |
|---|---|
| Source (natural) | Organism:  |
-Macromolecule #1: Actin, alpha skeletal muscle
| Macromolecule | Name: Actin, alpha skeletal muscle / type: protein_or_peptide / ID: 1 / Number of copies: 5 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  |
| Molecular weight | Theoretical: 41.875633 KDa |
| Sequence | String: DEDETTALVC DNGSGLVKAG FAGDDAPRAV FPSIVGRPRH QGVMVGMGQK DSYVGDEAQS KRGILTLKYP IE(HIC)GII TNW DDMEKIWHHT FYNELRVAPE EHPTLLTEAP LNPKANREKM TQIMFETFNV PAMYVAIQAV LSLYASGRTT GIVLDSG DG VTHNVPIYEG ...String: DEDETTALVC DNGSGLVKAG FAGDDAPRAV FPSIVGRPRH QGVMVGMGQK DSYVGDEAQS KRGILTLKYP IE(HIC)GII TNW DDMEKIWHHT FYNELRVAPE EHPTLLTEAP LNPKANREKM TQIMFETFNV PAMYVAIQAV LSLYASGRTT GIVLDSG DG VTHNVPIYEG YALPHAIMRL DLAGRDLTDY LMKILTERGY SFVTTAEREI VRDIKEKLCY VALDFENEMA TAASSSSL E KSYELPDGQV ITIGNERFRC PETLFQPSFI GMESAGIHET TYNSIMKCDI DIRKDLYANN VMSGGTTMYP GIADRMQKE ITALAPSTMK IKIIAPPERK YSVWIGGSIL ASLSTFQQMW ITKQEYDEAG PSIVHRKCF UniProtKB: Actin, alpha skeletal muscle |
-Macromolecule #2: PHOSPHOAMINOPHOSPHONIC ACID-ADENYLATE ESTER
| Macromolecule | Name: PHOSPHOAMINOPHOSPHONIC ACID-ADENYLATE ESTER / type: ligand / ID: 2 / Number of copies: 5 / Formula: ANP |
|---|---|
| Molecular weight | Theoretical: 506.196 Da |
| Chemical component information |  ChemComp-ANP: |
-Macromolecule #3: MAGNESIUM ION
| Macromolecule | Name: MAGNESIUM ION / type: ligand / ID: 3 / Number of copies: 5 / Formula: MG |
|---|---|
| Molecular weight | Theoretical: 24.305 Da |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | filament |
- Sample preparation
Sample preparation
| Buffer | pH: 7.5 Component:
Details: 5 mM HEPES pH 7.5, 0.1 M KCl, 2 mM MgCl2, 2 mM, 2 mM NaN3, 0.5 mM TCEP and 0.4 mM AppNHp. | |||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Grid | Model: Quantifoil R2/1 / Material: COPPER / Pretreatment - Type: GLOW DISCHARGE | |||||||||||||||||||||
| Vitrification | Cryogen name: ETHANE / Chamber humidity: 90 % / Chamber temperature: 298 K / Instrument: GATAN CRYOPLUNGE 3 Details: Manual backside blotting using Whatman filter paper No.5.. | |||||||||||||||||||||
| Details | Rise 27.36 A, Twist -166.58 degrees |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Specialist optics | Spherical aberration corrector: Cs-corrected microscope |
| Details | Cs-corrected microscope |
| Image recording | Film or detector model: FEI FALCON II (4k x 4k) / Detector mode: INTEGRATING / Digitization - Frames/image: 1-4 / Number real images: 2416 / Average exposure time: 1.5 sec. / Average electron dose: 110.0 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: SPOT SCAN / Imaging mode: BRIGHT FIELD |
| Sample stage | Cooling holder cryogen: NITROGEN |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
+ Image processing
Image processing
-Atomic model buiding 1
| Details | Rosetta iterative refinement was combined with MDFF. |
|---|---|
| Refinement | Space: REAL / Protocol: OTHER |
| Output model |  PDB-5ooe: |
 Movie
Movie Controller
Controller













 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)