+ Open data
Open data
- Basic information
Basic information
| Entry |  | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | Open Falcilysin, from MK-4815-treated dataset | |||||||||
 Map data Map data | ||||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | falcilysin open conformation / HYDROLASE | |||||||||
| Function / homology |  Function and homology information Function and homology informationhemoglobin catabolic process / apicoplast / food vacuole / Hydrolases; Acting on peptide bonds (peptidases); Metalloendopeptidases / vacuolar membrane / metalloendopeptidase activity / protein processing / metal ion binding Similarity search - Function | |||||||||
| Biological species |  | |||||||||
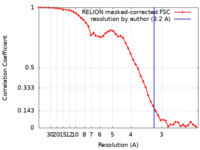
| Method | single particle reconstruction / cryo EM / Resolution: 3.2 Å | |||||||||
 Authors Authors | Lin JQ / Yan XF / Lescar J | |||||||||
| Funding support |  Singapore, 1 items Singapore, 1 items
| |||||||||
 Citation Citation |  Journal: Commun Biol / Year: 2024 Journal: Commun Biol / Year: 2024Title: Inhibition of falcilysin from Plasmodium falciparum by interference with its closed-to-open dynamic transition. Authors: Jianqing Lin / Xinfu Yan / Zara Chung / Chong Wai Liew / Abbas El Sahili / Evgeniya V Pechnikova / Peter R Preiser / Zbynek Bozdech / Yong-Gui Gao / Julien Lescar /   Abstract: In the absence of an efficacious vaccine, chemotherapy remains crucial to prevent and treat malaria. Given its key role in haemoglobin degradation, falcilysin constitutes an attractive target. Here, ...In the absence of an efficacious vaccine, chemotherapy remains crucial to prevent and treat malaria. Given its key role in haemoglobin degradation, falcilysin constitutes an attractive target. Here, we reveal the mechanism of enzymatic inhibition of falcilysin by MK-4815, an investigational new drug with potent antimalarial activity. Using X-ray crystallography, we determine two binary complexes of falcilysin in a closed state, bound with peptide substrates from the haemoglobin α and β chains respectively. An antiparallel β-sheet is formed between the substrate and enzyme, accounting for sequence-independent recognition at positions P2 and P1. In contrast, numerous contacts favor tyrosine and phenylalanine at the P1' position of the substrate. Cryo-EM studies reveal a majority of unbound falcilysin molecules adopting an open conformation. Addition of MK-4815 shifts about two-thirds of falcilysin molecules to a closed state. These structures give atomic level pictures of the proteolytic cycle, in which falcilysin interconverts between a closed state conducive to proteolysis, and an open conformation amenable to substrate diffusion and products release. MK-4815 and quinolines bind to an allosteric pocket next to a hinge region of falcilysin and hinders this dynamic transition. These data should inform the design of potent inhibitors of falcilysin to combat malaria. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Supplemental images |
|---|
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_37939.map.gz emd_37939.map.gz | 6 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-37939-v30.xml emd-37939-v30.xml emd-37939.xml emd-37939.xml | 18.8 KB 18.8 KB | Display Display |  EMDB header EMDB header |
| FSC (resolution estimation) |  emd_37939_fsc.xml emd_37939_fsc.xml | 4.6 KB | Display |  FSC data file FSC data file |
| Images |  emd_37939.png emd_37939.png | 19.2 KB | ||
| Masks |  emd_37939_msk_1.map emd_37939_msk_1.map | 8 MB |  Mask map Mask map | |
| Filedesc metadata |  emd-37939.cif.gz emd-37939.cif.gz | 6.1 KB | ||
| Others |  emd_37939_additional_1.map.gz emd_37939_additional_1.map.gz emd_37939_additional_2.map.gz emd_37939_additional_2.map.gz emd_37939_half_map_1.map.gz emd_37939_half_map_1.map.gz emd_37939_half_map_2.map.gz emd_37939_half_map_2.map.gz | 1.2 MB 7.5 MB 6 MB 6 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-37939 http://ftp.pdbj.org/pub/emdb/structures/EMD-37939 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-37939 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-37939 | HTTPS FTP |
-Validation report
| Summary document |  emd_37939_validation.pdf.gz emd_37939_validation.pdf.gz | 880.9 KB | Display |  EMDB validaton report EMDB validaton report |
|---|---|---|---|---|
| Full document |  emd_37939_full_validation.pdf.gz emd_37939_full_validation.pdf.gz | 880.4 KB | Display | |
| Data in XML |  emd_37939_validation.xml.gz emd_37939_validation.xml.gz | 10.1 KB | Display | |
| Data in CIF |  emd_37939_validation.cif.gz emd_37939_validation.cif.gz | 13.7 KB | Display | |
| Arichive directory |  https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-37939 https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-37939 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-37939 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-37939 | HTTPS FTP |
-Related structure data
| Related structure data |  8wyuMC  8wxwC  8wxzC  8wytC  8wyxC  8wyyC M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_37939.map.gz / Format: CCP4 / Size: 8 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_37939.map.gz / Format: CCP4 / Size: 8 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 1.16 Å | ||||||||||||||||||||||||||||||||||||
| Density |
| ||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
|
-Supplemental data
-Mask #1
| File |  emd_37939_msk_1.map emd_37939_msk_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Additional map: #2
| File | emd_37939_additional_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Additional map: #1
| File | emd_37939_additional_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Half map: #2
| File | emd_37939_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Half map: #1
| File | emd_37939_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
| Density Histograms |
- Sample components
Sample components
-Entire : Falcilysin
| Entire | Name: Falcilysin |
|---|---|
| Components |
|
-Supramolecule #1: Falcilysin
| Supramolecule | Name: Falcilysin / type: complex / ID: 1 / Parent: 0 / Macromolecule list: #1 |
|---|---|
| Source (natural) | Organism:  |
-Macromolecule #1: Falcilysin
| Macromolecule | Name: Falcilysin / type: protein_or_peptide / ID: 1 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  |
| Molecular weight | Theoretical: 132.034156 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: IHEKSPKHNS YDIIEKRYNE EFKMTYTVYQ HKKAKTQVIS LGTNDPLDVE QAFAFYVKTL THSGKGIPHI LEHSVLSGSK NYNYKNSIG LLEKGTLHTH LNAYTFNDRT VYMAGSMNNK DFFNIMGVYM DSVFQPNVLE NKYIFETEGW TYEVEKLKED E KGKAEIPQ ...String: IHEKSPKHNS YDIIEKRYNE EFKMTYTVYQ HKKAKTQVIS LGTNDPLDVE QAFAFYVKTL THSGKGIPHI LEHSVLSGSK NYNYKNSIG LLEKGTLHTH LNAYTFNDRT VYMAGSMNNK DFFNIMGVYM DSVFQPNVLE NKYIFETEGW TYEVEKLKED E KGKAEIPQ MKDYKVSFNG IVYNEMKGAL SSPLEDLYHE EMKYMFPDNV HSNNSGGDPK EITNLTYEEF KEFYYKNYNP KK VKVFFFS KNNPTELLNF VDQYLGQLDY SKYRDDAVES VEYQTYKKGP FYIKKKYGDH SEEKENLVSV AWLLNPKVDK TNN HNNNHS NNQSSENNGY SNGSHSSDLS LENPTDYFVL LIINNLLIHT PESVLYKALT DCGLGNNVID RGLNDSLVQY IFSI GLKGI KRNNEKIKNF DKVHYEVEDV IMNALKKVVK EGFNKSAVEA SINNIEFILK EANLKTSKSI DFVFEMTSKL NYNRD PLLI FEFEKYLNIV KNKIKNEPMY LEKFVEKHFI NNAHRSVILL EGDENYAQEQ ENLEKQELKK RIENFNEQEK EQVIKN FEE LSKYKNAEES PEHLNKFPII SISDLNKKTL EVPVNVYFTN INENNNIMET YNKLKTNEHM LKDNMDVFLK KYVLKND KH NTNNNNNNNN NMDYSFTETK YEGNVPILVY EMPTTGIVYL QFVFSLDHLT VDELAYLNLF KTLILENKTN KRSSEDFV I LREKNIGSMS ANVALYSKDD HLNVTDKYNA QALFNLEMHV LSHKCNDALN IALEAVKESD FSNKKKVIDI LKRKINGMK TTFSEKGYAI LMKYVKAHLN SKHYAHNIIY GYENYLKLQE QLELAENDFK TLENILVRIR NKIFNKKNLM VSVTSDYGAL KHLFVNSNE SLKNLVSYFE ENDKYINDMQ NKVNDPTVMG WNEEIKSKKL FDEEKVKKEF FVLPTFVNSV SMSGILFKPG E YLDPSFTV IVAALKNSYL WDTVRGLNGA YGVFADIEYD GSVVFLSARD PNLEKTLATF RESAKGLRKM ADTMTENDLL RY IINTIGT IDKPRRGIEL SKLSFLRLIS NESEQDRVEF RKRIMNTKKE DFYKFADLLE SKVNEFEKNI VIITTKEKAN EYI ANVDGE FKKVLIE UniProtKB: Falcilysin |
-Macromolecule #2: ZINC ION
| Macromolecule | Name: ZINC ION / type: ligand / ID: 2 / Number of copies: 1 / Formula: ZN |
|---|---|
| Molecular weight | Theoretical: 65.409 Da |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Concentration | 0.5 mg/mL |
|---|---|
| Buffer | pH: 7.5 / Details: 20 mM Na HEPES, 300 mM NaCl, 0.5 mM TCEP, pH 7.5 |
| Vitrification | Cryogen name: ETHANE / Chamber humidity: 100 % |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Image recording | Film or detector model: FEI FALCON IV (4k x 4k) / Average electron dose: 40.0 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD / Nominal defocus max: 2.5 µm / Nominal defocus min: 0.5 µm |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
 Movie
Movie Controller
Controller









 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)