[English] 日本語
 Yorodumi
Yorodumi- EMDB-35280: The focused refinement of CCT4-PhLP2A from TRiC-PhLP2A complex in... -
+ Open data
Open data
- Basic information
Basic information
| Entry |  | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | The focused refinement of CCT4-PhLP2A from TRiC-PhLP2A complex in the open state | |||||||||
 Map data Map data | A focused refined map of CCT4-PhLP2A | |||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | chaperonin complex / CHAPERONE / cochaperone | |||||||||
| Function / homology |  Function and homology information Function and homology information: / perinucleolar compartment / zona pellucida receptor complex / positive regulation of protein localization to Cajal body / scaRNA localization to Cajal body / positive regulation of telomerase RNA localization to Cajal body / chaperonin-containing T-complex / : / BBSome-mediated cargo-targeting to cilium / Formation of tubulin folding intermediates by CCT/TriC ...: / perinucleolar compartment / zona pellucida receptor complex / positive regulation of protein localization to Cajal body / scaRNA localization to Cajal body / positive regulation of telomerase RNA localization to Cajal body / chaperonin-containing T-complex / : / BBSome-mediated cargo-targeting to cilium / Formation of tubulin folding intermediates by CCT/TriC / binding of sperm to zona pellucida / Folding of actin by CCT/TriC / vascular endothelial growth factor receptor 2 binding / Prefoldin mediated transfer of substrate to CCT/TriC / regulation of peptidyl-tyrosine phosphorylation / Association of TriC/CCT with target proteins during biosynthesis / negative regulation of ubiquitin-dependent protein catabolic process / Hydrolases; Acting on acid anhydrides; In phosphorus-containing anhydrides / : / positive regulation of telomere maintenance via telomerase / positive regulation of endothelial cell proliferation / protein folding chaperone / cell projection / ATP-dependent protein folding chaperone / positive regulation of angiogenesis / unfolded protein binding / melanosome / protein folding / Cooperation of PDCL (PhLP1) and TRiC/CCT in G-protein beta folding / cell body / actin cytoskeleton organization / angiogenesis / microtubule / protein stabilization / apoptotic process / centrosome / positive regulation of gene expression / perinuclear region of cytoplasm / endoplasmic reticulum / ATP hydrolysis activity / protein-containing complex / RNA binding / extracellular exosome / nucleoplasm / ATP binding / cytosol / cytoplasm Similarity search - Function | |||||||||
| Biological species |  Homo sapiens (human) Homo sapiens (human) | |||||||||
| Method | single particle reconstruction / cryo EM / Resolution: 4.22 Å | |||||||||
 Authors Authors | Roh SH / Park J / Kim H / Lim S | |||||||||
| Funding support |  Korea, Republic Of, 1 items Korea, Republic Of, 1 items
| |||||||||
 Citation Citation |  Journal: Nat Commun / Year: 2024 Journal: Nat Commun / Year: 2024Title: A structural vista of phosducin-like PhLP2A-chaperonin TRiC cooperation during the ATP-driven folding cycle. Authors: Junsun Park / Hyunmin Kim / Daniel Gestaut / Seyeon Lim / Kwadwo A Opoku-Nsiah / Alexander Leitner / Judith Frydman / Soung-Hun Roh /    Abstract: Proper cellular proteostasis, essential for viability, requires a network of chaperones and cochaperones. ATP-dependent chaperonin TRiC/CCT partners with cochaperones prefoldin (PFD) and phosducin- ...Proper cellular proteostasis, essential for viability, requires a network of chaperones and cochaperones. ATP-dependent chaperonin TRiC/CCT partners with cochaperones prefoldin (PFD) and phosducin-like proteins (PhLPs) to facilitate folding of essential eukaryotic proteins. Using cryoEM and biochemical analyses, we determine the ATP-driven cycle of TRiC-PFD-PhLP2A interaction. PhLP2A binds to open apo-TRiC through polyvalent domain-specific contacts with its chamber's equatorial and apical regions. PhLP2A N-terminal H3-domain binding to subunits CCT3/4 apical domains displace PFD from TRiC. ATP-induced TRiC closure rearranges the contacts of PhLP2A domains within the closed chamber. In the presence of substrate, actin and PhLP2A segregate into opposing chambers, each binding to positively charged inner surface residues from CCT1/3/6/8. Notably, actin induces a conformational change in PhLP2A, causing its N-terminal helices to extend across the inter-ring interface to directly contact a hydrophobic groove in actin. Our findings reveal an ATP-driven PhLP2A structural rearrangement cycle within the TRiC chamber to facilitate folding. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Supplemental images |
|---|
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_35280.map.gz emd_35280.map.gz | 117.7 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-35280-v30.xml emd-35280-v30.xml emd-35280.xml emd-35280.xml | 21.1 KB 21.1 KB | Display Display |  EMDB header EMDB header |
| FSC (resolution estimation) |  emd_35280_fsc.xml emd_35280_fsc.xml | 10.6 KB | Display |  FSC data file FSC data file |
| Images |  emd_35280.png emd_35280.png | 43 KB | ||
| Filedesc metadata |  emd-35280.cif.gz emd-35280.cif.gz | 6.8 KB | ||
| Others |  emd_35280_half_map_1.map.gz emd_35280_half_map_1.map.gz emd_35280_half_map_2.map.gz emd_35280_half_map_2.map.gz | 116 MB 116 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-35280 http://ftp.pdbj.org/pub/emdb/structures/EMD-35280 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-35280 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-35280 | HTTPS FTP |
-Validation report
| Summary document |  emd_35280_validation.pdf.gz emd_35280_validation.pdf.gz | 670.2 KB | Display |  EMDB validaton report EMDB validaton report |
|---|---|---|---|---|
| Full document |  emd_35280_full_validation.pdf.gz emd_35280_full_validation.pdf.gz | 669.8 KB | Display | |
| Data in XML |  emd_35280_validation.xml.gz emd_35280_validation.xml.gz | 18.7 KB | Display | |
| Data in CIF |  emd_35280_validation.cif.gz emd_35280_validation.cif.gz | 24.2 KB | Display | |
| Arichive directory |  https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-35280 https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-35280 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-35280 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-35280 | HTTPS FTP |
-Related structure data
| Related structure data |  8i9qMC  8i1uC  8i6jC  8i9uC  8ib8C C: citing same article ( M: atomic model generated by this map |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_35280.map.gz / Format: CCP4 / Size: 125 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_35280.map.gz / Format: CCP4 / Size: 125 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | A focused refined map of CCT4-PhLP2A | ||||||||||||||||||||||||||||||||||||
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 1.13 Å | ||||||||||||||||||||||||||||||||||||
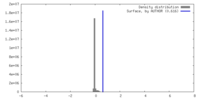
| Density |
| ||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
|
-Supplemental data
-Half map: A focused refined half map of CCT4-PhLP2A
| File | emd_35280_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | A focused refined half map of CCT4-PhLP2A | ||||||||||||
| Projections & Slices |
| ||||||||||||
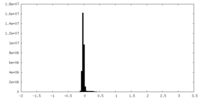
| Density Histograms |
-Half map: A focused refined half map of CCT4-PhLP2A
| File | emd_35280_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | A focused refined half map of CCT4-PhLP2A | ||||||||||||
| Projections & Slices |
| ||||||||||||
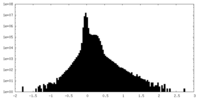
| Density Histograms |
- Sample components
Sample components
-Entire : Complex of human TRiC/CCT-PhLP2A
| Entire | Name: Complex of human TRiC/CCT-PhLP2A |
|---|---|
| Components |
|
-Supramolecule #1: Complex of human TRiC/CCT-PhLP2A
| Supramolecule | Name: Complex of human TRiC/CCT-PhLP2A / type: complex / ID: 1 / Parent: 0 / Macromolecule list: all |
|---|---|
| Molecular weight | Theoretical: 1 MDa |
-Supramolecule #2: T-complex protein 1 subunit delta from human TRiC/CCT complex
| Supramolecule | Name: T-complex protein 1 subunit delta from human TRiC/CCT complex type: complex / ID: 2 / Parent: 1 / Macromolecule list: #1 |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
-Supramolecule #3: PhLP2A (PDCL3)
| Supramolecule | Name: PhLP2A (PDCL3) / type: complex / ID: 3 / Parent: 1 / Macromolecule list: #2 |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
-Macromolecule #1: T-complex protein 1 subunit delta
| Macromolecule | Name: T-complex protein 1 subunit delta / type: protein_or_peptide / ID: 1 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 57.996113 KDa |
| Recombinant expression | Organism:  Trichoplusia ni (cabbage looper) Trichoplusia ni (cabbage looper) |
| Sequence | String: MPENVAPRSG ATAGAAGGRG KGAYQDRDKP AQIRFSNISA AKAVADAIRT SLGPKGMDKM IQDGKGDVTI TNDGATILKQ MQVLHPAAR MLVELSKAQD IEAGDGTTSV VIIAGSLLDS CTKLLQKGIH PTIISESFQK ALEKGIEILT DMSRPVELSD R ETLLNSAT ...String: MPENVAPRSG ATAGAAGGRG KGAYQDRDKP AQIRFSNISA AKAVADAIRT SLGPKGMDKM IQDGKGDVTI TNDGATILKQ MQVLHPAAR MLVELSKAQD IEAGDGTTSV VIIAGSLLDS CTKLLQKGIH PTIISESFQK ALEKGIEILT DMSRPVELSD R ETLLNSAT TSLNSKVVSQ YSSLLSPMSV NAVMKVIDPA TATSVDLRDI KIVKKLGGTI DDCELVEGLV LTQKVSNSGI TR VEKAKIG LIQFCLSAPK TDMDNQIVVS DYAQMDRVLR EERAYILNLV KQIKKTGCNV LLIQKSILRD ALSDLALHFL NKM KIMVIK DIEREDIEFI CKTIGTKPVA HIDQFTADML GSAELAEEVN LNGSGKLLKI TGCASPGKTV TIVVRGSNKL VIEE AERSI HDALCVIRCL VKKRALIAGG GAPEIELALR LTEYSRTLSG MESYCVRAFA DAMEVIPSTL AENAGLNPIS TVTEL RNRH AQGEKTAGIN VRKGGISNIL EELVVQPLLV SVSALTLATE TVRSILKIDD VVNTR UniProtKB: T-complex protein 1 subunit delta |
-Macromolecule #2: Phosducin-like protein 3
| Macromolecule | Name: Phosducin-like protein 3 / type: protein_or_peptide / ID: 2 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 27.650383 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: MQDPNADTEW NDILRKKGIL PPKESLKELE EEAEEEQRIL QQSVVKTYED MTLEELEDHE DEFNEEDERA IEMYRRRRLA EWKATKLKN KFGEVLEISG KDYVQEVTKA GEGLWVILHL YKQGIPLCAL INQHLSGLAR KFPDVKFIKA ISTTCIPNYP D RNLPTIFV ...String: MQDPNADTEW NDILRKKGIL PPKESLKELE EEAEEEQRIL QQSVVKTYED MTLEELEDHE DEFNEEDERA IEMYRRRRLA EWKATKLKN KFGEVLEISG KDYVQEVTKA GEGLWVILHL YKQGIPLCAL INQHLSGLAR KFPDVKFIKA ISTTCIPNYP D RNLPTIFV YLEGDIKAQF IGPLVFGGMN LTRDELEWKL SESGAIMTDL EENPKKPIED VLLSSVRRSV LMKRDSDSEG D UniProtKB: Phosducin-like protein 3 |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | 2D array |
- Sample preparation
Sample preparation
| Buffer | pH: 7.4 Component:
| |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Grid | Model: Quantifoil R1.2/1.3 / Material: COPPER / Support film - Material: CARBON / Support film - topology: HOLEY | |||||||||
| Vitrification | Cryogen name: ETHANE / Chamber humidity: 100 % / Instrument: FEI VITROBOT MARK IV |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Image recording | Film or detector model: FEI FALCON IV (4k x 4k) / Detector mode: COUNTING / Digitization - Dimensions - Width: 4096 pixel / Digitization - Dimensions - Height: 4096 pixel / Number real images: 15075 / Average electron dose: 50.0 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD / Cs: 2.7 mm / Nominal defocus max: 2.0 µm / Nominal defocus min: 1.0 µm |
| Sample stage | Specimen holder model: FEI TITAN KRIOS AUTOGRID HOLDER / Cooling holder cryogen: NITROGEN |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
 Movie
Movie Controller
Controller














 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)