[English] 日本語
 Yorodumi
Yorodumi- EMDB-35199: The focused refinement of CCT3-PhLP2A from TRiC-PhLP2A complex in... -
+ Open data
Open data
- Basic information
Basic information
| Entry |  | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | The focused refinement of CCT3-PhLP2A from TRiC-PhLP2A complex in the open state | |||||||||
 Map data Map data | ||||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | chaperonin complex / CHAPERONE / cochaperone | |||||||||
| Function / homology |  Function and homology information Function and homology information: / perinucleolar compartment / zona pellucida receptor complex / positive regulation of protein localization to Cajal body / positive regulation of telomerase RNA localization to Cajal body / chaperonin-containing T-complex / BBSome-mediated cargo-targeting to cilium / Formation of tubulin folding intermediates by CCT/TriC / binding of sperm to zona pellucida / Folding of actin by CCT/TriC ...: / perinucleolar compartment / zona pellucida receptor complex / positive regulation of protein localization to Cajal body / positive regulation of telomerase RNA localization to Cajal body / chaperonin-containing T-complex / BBSome-mediated cargo-targeting to cilium / Formation of tubulin folding intermediates by CCT/TriC / binding of sperm to zona pellucida / Folding of actin by CCT/TriC / vascular endothelial growth factor receptor 2 binding / Prefoldin mediated transfer of substrate to CCT/TriC / regulation of peptidyl-tyrosine phosphorylation / : / Association of TriC/CCT with target proteins during biosynthesis / negative regulation of ubiquitin-dependent protein catabolic process / Hydrolases; Acting on acid anhydrides; In phosphorus-containing anhydrides / positive regulation of endothelial cell proliferation / positive regulation of telomere maintenance via telomerase / protein folding chaperone / ATP-dependent protein folding chaperone / positive regulation of angiogenesis / unfolded protein binding / Cooperation of PDCL (PhLP1) and TRiC/CCT in G-protein beta folding / protein folding / actin cytoskeleton organization / cell body / angiogenesis / microtubule / cytoskeleton / protein stabilization / apoptotic process / positive regulation of gene expression / perinuclear region of cytoplasm / endoplasmic reticulum / ATP hydrolysis activity / protein-containing complex / RNA binding / extracellular exosome / nucleoplasm / ATP binding / cytoplasm / cytosol Similarity search - Function | |||||||||
| Biological species |  Homo sapiens (human) Homo sapiens (human) | |||||||||
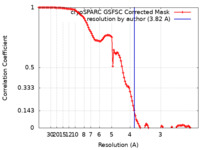
| Method | single particle reconstruction / cryo EM / Resolution: 3.82 Å | |||||||||
 Authors Authors | Roh SH / Park J / Kim H / Lim S | |||||||||
| Funding support |  Korea, Republic Of, 1 items Korea, Republic Of, 1 items
| |||||||||
 Citation Citation |  Journal: Nat Commun / Year: 2024 Journal: Nat Commun / Year: 2024Title: A structural vista of phosducin-like PhLP2A-chaperonin TRiC cooperation during the ATP-driven folding cycle. Authors: Junsun Park / Hyunmin Kim / Daniel Gestaut / Seyeon Lim / Kwadwo A Opoku-Nsiah / Alexander Leitner / Judith Frydman / Soung-Hun Roh /    Abstract: Proper cellular proteostasis, essential for viability, requires a network of chaperones and cochaperones. ATP-dependent chaperonin TRiC/CCT partners with cochaperones prefoldin (PFD) and phosducin- ...Proper cellular proteostasis, essential for viability, requires a network of chaperones and cochaperones. ATP-dependent chaperonin TRiC/CCT partners with cochaperones prefoldin (PFD) and phosducin-like proteins (PhLPs) to facilitate folding of essential eukaryotic proteins. Using cryoEM and biochemical analyses, we determine the ATP-driven cycle of TRiC-PFD-PhLP2A interaction. PhLP2A binds to open apo-TRiC through polyvalent domain-specific contacts with its chamber's equatorial and apical regions. PhLP2A N-terminal H3-domain binding to subunits CCT3/4 apical domains displace PFD from TRiC. ATP-induced TRiC closure rearranges the contacts of PhLP2A domains within the closed chamber. In the presence of substrate, actin and PhLP2A segregate into opposing chambers, each binding to positively charged inner surface residues from CCT1/3/6/8. Notably, actin induces a conformational change in PhLP2A, causing its N-terminal helices to extend across the inter-ring interface to directly contact a hydrophobic groove in actin. Our findings reveal an ATP-driven PhLP2A structural rearrangement cycle within the TRiC chamber to facilitate folding. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Supplemental images |
|---|
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_35199.map.gz emd_35199.map.gz | 117.8 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-35199-v30.xml emd-35199-v30.xml emd-35199.xml emd-35199.xml | 21.4 KB 21.4 KB | Display Display |  EMDB header EMDB header |
| FSC (resolution estimation) |  emd_35199_fsc.xml emd_35199_fsc.xml | 10.6 KB | Display |  FSC data file FSC data file |
| Images |  emd_35199.png emd_35199.png | 52.8 KB | ||
| Filedesc metadata |  emd-35199.cif.gz emd-35199.cif.gz | 6.9 KB | ||
| Others |  emd_35199_half_map_1.map.gz emd_35199_half_map_1.map.gz emd_35199_half_map_2.map.gz emd_35199_half_map_2.map.gz | 116 MB 116 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-35199 http://ftp.pdbj.org/pub/emdb/structures/EMD-35199 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-35199 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-35199 | HTTPS FTP |
-Related structure data
| Related structure data |  8i6jMC  8i1uC  8i9qC  8i9uC  8ib8C C: citing same article ( M: atomic model generated by this map |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_35199.map.gz / Format: CCP4 / Size: 125 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_35199.map.gz / Format: CCP4 / Size: 125 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 1.13 Å | ||||||||||||||||||||||||||||||||||||
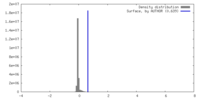
| Density |
| ||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
|
-Supplemental data
-Half map: A half map for PhLP2A-CCT3 open TRiC focused map
| File | emd_35199_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | A half map for PhLP2A-CCT3 open TRiC focused map | ||||||||||||
| Projections & Slices |
| ||||||||||||
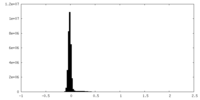
| Density Histograms |
-Half map: A half map for PhLP2A-CCT3 open TRiC focused map
| File | emd_35199_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | A half map for PhLP2A-CCT3 open TRiC focused map | ||||||||||||
| Projections & Slices |
| ||||||||||||
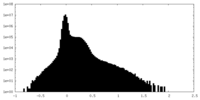
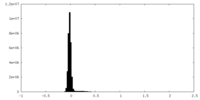
| Density Histograms |
- Sample components
Sample components
-Entire : Complex of TRiC/CCT and PhLP2A
| Entire | Name: Complex of TRiC/CCT and PhLP2A |
|---|---|
| Components |
|
-Supramolecule #1: Complex of TRiC/CCT and PhLP2A
| Supramolecule | Name: Complex of TRiC/CCT and PhLP2A / type: complex / ID: 1 / Parent: 0 / Macromolecule list: #1-#2 |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 1 MDa |
-Supramolecule #2: PhLP2A (PDCL3)
| Supramolecule | Name: PhLP2A (PDCL3) / type: complex / ID: 2 / Parent: 1 / Macromolecule list: #1 |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
-Supramolecule #3: TRiC/CCT
| Supramolecule | Name: TRiC/CCT / type: complex / ID: 3 / Parent: 1 / Macromolecule list: #2 |
|---|
-Macromolecule #1: Phosducin-like protein 3
| Macromolecule | Name: Phosducin-like protein 3 / type: protein_or_peptide / ID: 1 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 27.650383 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: MQDPNADTEW NDILRKKGIL PPKESLKELE EEAEEEQRIL QQSVVKTYED MTLEELEDHE DEFNEEDERA IEMYRRRRLA EWKATKLKN KFGEVLEISG KDYVQEVTKA GEGLWVILHL YKQGIPLCAL INQHLSGLAR KFPDVKFIKA ISTTCIPNYP D RNLPTIFV ...String: MQDPNADTEW NDILRKKGIL PPKESLKELE EEAEEEQRIL QQSVVKTYED MTLEELEDHE DEFNEEDERA IEMYRRRRLA EWKATKLKN KFGEVLEISG KDYVQEVTKA GEGLWVILHL YKQGIPLCAL INQHLSGLAR KFPDVKFIKA ISTTCIPNYP D RNLPTIFV YLEGDIKAQF IGPLVFGGMN LTRDELEWKL SESGAIMTDL EENPKKPIED VLLSSVRRSV LMKRDSDSEG D UniProtKB: Phosducin-like protein 3 |
-Macromolecule #2: T-complex protein 1 subunit gamma
| Macromolecule | Name: T-complex protein 1 subunit gamma / type: protein_or_peptide / ID: 2 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 60.613855 KDa |
| Recombinant expression | Organism:  Trichoplusia ni (cabbage looper) Trichoplusia ni (cabbage looper) |
| Sequence | String: MMGHRPVLVL SQNTKRESGR KVQSGNINAA KTIADIIRTC LGPKSMMKML LDPMGGIVMT NDGNAILREI QVQHPAAKSM IEISRTQDE EVGDGTTSVI ILAGEMLSVA EHFLEQQMHP TVVISAYRKA LDDMISTLKK ISIPVDISDS DMMLNIINSS I TTKAISRW ...String: MMGHRPVLVL SQNTKRESGR KVQSGNINAA KTIADIIRTC LGPKSMMKML LDPMGGIVMT NDGNAILREI QVQHPAAKSM IEISRTQDE EVGDGTTSVI ILAGEMLSVA EHFLEQQMHP TVVISAYRKA LDDMISTLKK ISIPVDISDS DMMLNIINSS I TTKAISRW SSLACNIALD AVKMVQFEEN GRKEIDIKKY ARVEKIPGGI IEDSCVLRGV MINKDVTHPR MRRYIKNPRI VL LDSSLEY KKGESQTDIE ITREEDFTRI LQMEEEYIQQ LCEDIIQLKP DVVITEKGIS DLAQHYLMRA NITAIRRVRK TDN NRIARA CGARIVSRPE ELREDDVGTG AGLLEIKKIG DEYFTFITDC KDPKACTILL RGASKEILSE VERNLQDAMQ VCRN VLLDP QLVPGGGASE MAVAHALTEK SKAMTGVEQW PYRAVAQALE VIPRTLIQNC GASTIRLLTS LRAKHTQENC ETWGV NGET GTLVDMKELG IWEPLAVKLQ TYKTAVETAV LLLRIDDIVS GHKKKGDDQS RQGGAPDAGQ E UniProtKB: T-complex protein 1 subunit gamma |
-Macromolecule #3: ADENOSINE-5'-DIPHOSPHATE
| Macromolecule | Name: ADENOSINE-5'-DIPHOSPHATE / type: ligand / ID: 3 / Number of copies: 1 / Formula: ADP |
|---|---|
| Molecular weight | Theoretical: 427.201 Da |
| Chemical component information |  ChemComp-ADP: |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | 2D array |
- Sample preparation
Sample preparation
| Buffer | pH: 7.4 Component:
| |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Grid | Model: Quantifoil R1.2/1.3 / Support film - Material: CARBON / Support film - topology: HOLEY | |||||||||
| Vitrification | Cryogen name: ETHANE / Chamber humidity: 100 % / Instrument: FEI VITROBOT MARK IV |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Image recording | Film or detector model: FEI FALCON IV (4k x 4k) / Detector mode: COUNTING / Digitization - Dimensions - Width: 4096 pixel / Digitization - Dimensions - Height: 4096 pixel / Number real images: 15075 / Average electron dose: 50.0 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD / Cs: 2.7 mm / Nominal defocus max: 2.0 µm / Nominal defocus min: 1.0 µm |
| Sample stage | Specimen holder model: FEI TITAN KRIOS AUTOGRID HOLDER / Cooling holder cryogen: NITROGEN |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
 Movie
Movie Controller
Controller














 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)