[English] 日本語
 Yorodumi
Yorodumi- EMDB-35230: In situ structure of axonemal doublet microtubules in mouse sperm... -
+ Open data
Open data
- Basic information
Basic information
| Entry |  | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | In situ structure of axonemal doublet microtubules in mouse sperm with 48-nm repeat | |||||||||
 Map data Map data | In situ structure of axonemal doublet microtubules in mouse sperm with 48-nm repeat | |||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | microtubules / axoneme / sperm / filament / STRUCTURAL PROTEIN | |||||||||
| Function / homology |  Function and homology information Function and homology informationmale germ-line stem cell population maintenance / protein localization to motile cilium / left/right pattern formation / axonemal microtubule doublet inner sheath / outer acrosomal membrane / epithelial cilium movement involved in determination of left/right asymmetry / regulation of brood size / establishment of left/right asymmetry / 9+0 motile cilium / Microtubule-dependent trafficking of connexons from Golgi to the plasma membrane ...male germ-line stem cell population maintenance / protein localization to motile cilium / left/right pattern formation / axonemal microtubule doublet inner sheath / outer acrosomal membrane / epithelial cilium movement involved in determination of left/right asymmetry / regulation of brood size / establishment of left/right asymmetry / 9+0 motile cilium / Microtubule-dependent trafficking of connexons from Golgi to the plasma membrane / Cilium Assembly / Sealing of the nuclear envelope (NE) by ESCRT-III / sperm flagellum assembly / manchette assembly / axonemal B tubule inner sheath / axonemal A tubule inner sheath / Carboxyterminal post-translational modifications of tubulin / Intraflagellar transport / protein polyglutamylation / regulation of calcineurin-NFAT signaling cascade / sperm axoneme assembly / regulation of microtubule nucleation / positive regulation of feeding behavior / COPI-independent Golgi-to-ER retrograde traffic / Transferases; Transferring phosphorus-containing groups / HSP90 chaperone cycle for steroid hormone receptors (SHR) in the presence of ligand / MAP kinase tyrosine/serine/threonine phosphatase activity / inner dynein arm assembly / sperm principal piece / cerebrospinal fluid circulation / regulation of cilium beat frequency involved in ciliary motility / epithelial cilium movement involved in extracellular fluid movement / COPI-mediated anterograde transport / Aggrephagy / 9+2 motile cilium / Kinesins / Mitotic Prometaphase / EML4 and NUDC in mitotic spindle formation / protein localization to organelle / cilium movement involved in cell motility / intraciliary transport / regulation of store-operated calcium entry / Resolution of Sister Chromatid Cohesion / PKR-mediated signaling / The role of GTSE1 in G2/M progression after G2 checkpoint / acrosomal membrane / RHO GTPases activate IQGAPs / Recycling pathway of L1 / axoneme assembly / ciliary transition zone / left/right axis specification / cilium movement / microtubule sliding / COPI-dependent Golgi-to-ER retrograde traffic / axonemal microtubule / RHO GTPases Activate Formins / calcium ion sensor activity / Separation of Sister Chromatids / Hedgehog 'off' state / cilium organization / Loss of Nlp from mitotic centrosomes / Recruitment of mitotic centrosome proteins and complexes / Loss of proteins required for interphase microtubule organization from the centrosome / Recruitment of NuMA to mitotic centrosomes / Anchoring of the basal body to the plasma membrane / AURKA Activation by TPX2 / gamma-tubulin ring complex / Regulation of PLK1 Activity at G2/M Transition / manchette / positive regulation of cilium assembly / MHC class II antigen presentation / flagellated sperm motility / cell projection organization / UTP biosynthetic process / CTP biosynthetic process / determination of left/right symmetry / intermediate filament / protein targeting to membrane / extrinsic component of membrane / nucleoside diphosphate kinase activity / positive regulation of cell motility / GTP biosynthetic process / : / tubulin complex / AMP binding / protein-serine/threonine phosphatase / receptor clustering / ciliary base / protein serine/threonine phosphatase activity / phosphatase activity / microtubule organizing center / phosphoprotein phosphatase activity / mitotic cytokinesis / cellular response to UV-C / regulation of cell division / cilium assembly / glial cell projection / axoneme / cellular response to unfolded protein / spermatid development Similarity search - Function | |||||||||
| Biological species |  | |||||||||
| Method | subtomogram averaging / cryo EM / Resolution: 6.5 Å | |||||||||
 Authors Authors | Zhu Y / Yin GL / Tai LH / Sun F | |||||||||
| Funding support |  China, 1 items China, 1 items
| |||||||||
 Citation Citation |  Journal: Cell Discov / Year: 2023 Journal: Cell Discov / Year: 2023Title: In-cell structural insight into the stability of sperm microtubule doublet. Authors: Linhua Tai / Guoliang Yin / Xiaojun Huang / Fei Sun / Yun Zhu /  Abstract: The propulsion for mammalian sperm swimming is generated by flagella beating. Microtubule doublets (DMTs) along with microtubule inner proteins (MIPs) are essential structural blocks of flagella. ...The propulsion for mammalian sperm swimming is generated by flagella beating. Microtubule doublets (DMTs) along with microtubule inner proteins (MIPs) are essential structural blocks of flagella. However, the intricate molecular architecture of intact sperm DMT remains elusive. Here, by in situ cryo-electron tomography, we solved the in-cell structure of mouse sperm DMT at 4.5-7.5 Å resolutions, and built its model with 36 kinds of MIPs in 48 nm periodicity. We identified multiple copies of Tektin5 that reinforce Tektin bundle, and multiple MIPs with different periodicities that anchor the Tektin bundle to tubulin wall. This architecture contributes to a superior stability of A-tubule than B-tubule of DMT, which was revealed by structural comparison of DMTs from the intact and deformed axonemes. Our work provides an overall molecular picture of intact sperm DMT in 48 nm periodicity that is essential to understand the molecular mechanism of sperm motility as well as the related ciliopathies. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Supplemental images |
|---|
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_35230.map.gz emd_35230.map.gz | 56.3 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-35230-v30.xml emd-35230-v30.xml emd-35230.xml emd-35230.xml | 61.9 KB 61.9 KB | Display Display |  EMDB header EMDB header |
| Images |  emd_35230.png emd_35230.png | 165.9 KB | ||
| Filedesc metadata |  emd-35230.cif.gz emd-35230.cif.gz | 17.2 KB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-35230 http://ftp.pdbj.org/pub/emdb/structures/EMD-35230 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-35230 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-35230 | HTTPS FTP |
-Related structure data
| Related structure data |  8i7rMC  8i7oC C: citing same article ( M: atomic model generated by this map |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_35230.map.gz / Format: CCP4 / Size: 125 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_35230.map.gz / Format: CCP4 / Size: 125 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | In situ structure of axonemal doublet microtubules in mouse sperm with 48-nm repeat | ||||||||||||||||||||||||||||||||||||
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 1.76 Å | ||||||||||||||||||||||||||||||||||||
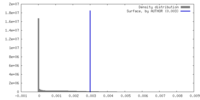
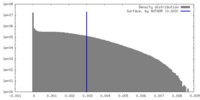
| Density |
| ||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
|
-Supplemental data
- Sample components
Sample components
+Entire : mouse sperm
+Supramolecule #1: mouse sperm
+Macromolecule #1: Meiosis-specific nuclear structural protein 1
+Macromolecule #2: Tektin-1
+Macromolecule #3: Tubulin beta-4B chain
+Macromolecule #4: Detyrosinated tubulin alpha-3 chain
+Macromolecule #5: Tektin-2
+Macromolecule #6: Nucleoside diphosphate kinase 7
+Macromolecule #7: Tektin-3
+Macromolecule #8: Tektin-4
+Macromolecule #9: EF-hand domain-containing family member B
+Macromolecule #10: Tektin bundle-interacting protein 1
+Macromolecule #11: Tektin-5
+Macromolecule #12: Cilia- and flagella-associated protein 53
+Macromolecule #13: EF-hand domain-containing protein 1
+Macromolecule #14: EF-hand domain-containing family member C2
+Macromolecule #15: Protein FAM166A
+Macromolecule #16: Cilia- and flagella-associated protein 95
+Macromolecule #17: Protein FAM166C
+Macromolecule #18: Cilia- and flagella-associated protein 107
+Macromolecule #19: Dual specificity phosphatase 21
+Macromolecule #20: Cilia- and flagella-associated protein 161
+Macromolecule #21: Coiled-coil domain-containing protein 105
+Macromolecule #22: Enkurin
+Macromolecule #23: Piercer of microtubule wall 1 protein
+Macromolecule #24: Testis-expressed protein 43
+Macromolecule #25: Piercer of microtubule wall 2 protein
+Macromolecule #26: Cilia- and flagella-associated protein 276
+Macromolecule #27: RIB43A-like with coiled-coils protein 2
+Macromolecule #28: Protein Flattop
+Macromolecule #29: Cilia- and flagella-associated protein 52
+Macromolecule #30: EF-hand calcium-binding domain-containing protein 6
+Macromolecule #31: Cilia and flagella-associated protein 77
+Macromolecule #32: Sperm-associated antigen 8
+Macromolecule #33: Cilia- and flagella-associated protein 45
+Macromolecule #34: Cilia- and flagella-associated protein 20
+Macromolecule #35: Parkin coregulated gene protein homolog
+Macromolecule #36: Cilia- and flagella-associated protein 210
+Macromolecule #37: Sperm acrosome-associated protein 9
+Macromolecule #38: Cilia- and flagella-associated protein 141
+Macromolecule #39: GUANOSINE-5'-TRIPHOSPHATE
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | subtomogram averaging |
| Aggregation state | cell |
- Sample preparation
Sample preparation
| Buffer | pH: 7 |
|---|---|
| Vitrification | Cryogen name: ETHANE |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Image recording | Film or detector model: GATAN K2 QUANTUM (4k x 4k) / Detector mode: SUPER-RESOLUTION / Average electron dose: 3.0 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: SPOT SCAN / Imaging mode: BRIGHT FIELD / Cs: 2.7 mm / Nominal defocus max: 5.0 µm / Nominal defocus min: 1.0 µm |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
- Image processing
Image processing
| Final reconstruction | Applied symmetry - Point group: C1 (asymmetric) / Resolution.type: BY AUTHOR / Resolution: 6.5 Å / Resolution method: FSC 0.143 CUT-OFF / Number subtomograms used: 17450 |
|---|---|
| Extraction | Number tomograms: 689 / Number images used: 17450 |
| CTF correction | Type: PHASE FLIPPING AND AMPLITUDE CORRECTION |
| Final angle assignment | Type: MAXIMUM LIKELIHOOD |
 Movie
Movie Controller
Controller




































 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)