+ Open data
Open data
- Basic information
Basic information
| Entry |  | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | SARS-CoV-2 Delta variant spike protein | |||||||||
 Map data Map data | ||||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | SARS-CoV-2 / Delta / Spike protein / VIRAL PROTEIN | |||||||||
| Function / homology |  Function and homology information Function and homology informationsymbiont-mediated disruption of host tissue / Maturation of spike protein / Translation of Structural Proteins / Virion Assembly and Release / host cell surface / host extracellular space / viral translation / symbiont-mediated-mediated suppression of host tetherin activity / Induction of Cell-Cell Fusion / structural constituent of virion ...symbiont-mediated disruption of host tissue / Maturation of spike protein / Translation of Structural Proteins / Virion Assembly and Release / host cell surface / host extracellular space / viral translation / symbiont-mediated-mediated suppression of host tetherin activity / Induction of Cell-Cell Fusion / structural constituent of virion / entry receptor-mediated virion attachment to host cell / membrane fusion / Attachment and Entry / host cell endoplasmic reticulum-Golgi intermediate compartment membrane / positive regulation of viral entry into host cell / receptor-mediated virion attachment to host cell / host cell surface receptor binding / symbiont-mediated suppression of host innate immune response / receptor ligand activity / endocytosis involved in viral entry into host cell / fusion of virus membrane with host plasma membrane / fusion of virus membrane with host endosome membrane / viral envelope / symbiont entry into host cell / virion attachment to host cell / SARS-CoV-2 activates/modulates innate and adaptive immune responses / host cell plasma membrane / virion membrane / identical protein binding / membrane / plasma membrane Similarity search - Function | |||||||||
| Biological species |  | |||||||||
| Method | single particle reconstruction / cryo EM / Resolution: 2.9 Å | |||||||||
 Authors Authors | Xu J / Cheng H / Liu N / Wang HW | |||||||||
| Funding support |  China, 1 items China, 1 items
| |||||||||
 Citation Citation |  Journal: Nat Commun / Year: 2024 Journal: Nat Commun / Year: 2024Title: Self-assembled superstructure alleviates air-water interface effect in cryo-EM. Authors: Liming Zheng / Jie Xu / Weihua Wang / Xiaoyin Gao / Chao Zhao / Weijun Guo / Luzhao Sun / Hang Cheng / Fanhao Meng / Buhang Chen / Weiyu Sun / Xia Jia / Xiong Zhou / Kai Wu / Zhongfan Liu / ...Authors: Liming Zheng / Jie Xu / Weihua Wang / Xiaoyin Gao / Chao Zhao / Weijun Guo / Luzhao Sun / Hang Cheng / Fanhao Meng / Buhang Chen / Weiyu Sun / Xia Jia / Xiong Zhou / Kai Wu / Zhongfan Liu / Feng Ding / Nan Liu / Hong-Wei Wang / Hailin Peng /  Abstract: Cryo-electron microscopy (cryo-EM) has been widely used to reveal the structures of proteins at atomic resolution. One key challenge is that almost all proteins are predominantly adsorbed to the air- ...Cryo-electron microscopy (cryo-EM) has been widely used to reveal the structures of proteins at atomic resolution. One key challenge is that almost all proteins are predominantly adsorbed to the air-water interface during standard cryo-EM specimen preparation. The interaction of proteins with air-water interface will significantly impede the success of reconstruction and achievable resolution. Here, we highlight the critical role of impenetrable surfactant monolayers in passivating the air-water interface problems, and develop a robust effective method for high-resolution cryo-EM analysis, by using the superstructure GSAMs which comprises surfactant self-assembled monolayers (SAMs) and graphene membrane. The GSAMs works well in enriching the orientations and improving particle utilization ratio of multiple proteins, facilitating the 3.3-Å resolution reconstruction of a 100-kDa protein complex (ACE2-RBD), which shows strong preferential orientation using traditional specimen preparation protocol. Additionally, we demonstrate that GSAMs enables the successful determinations of small proteins (<100 kDa) at near-atomic resolution. This study expands the understanding of SAMs and provides a key to better control the interaction of protein with air-water interface. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Supplemental images |
|---|
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_34974.map.gz emd_34974.map.gz | 483.8 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-34974-v30.xml emd-34974-v30.xml emd-34974.xml emd-34974.xml | 15.4 KB 15.4 KB | Display Display |  EMDB header EMDB header |
| Images |  emd_34974.png emd_34974.png | 83.1 KB | ||
| Filedesc metadata |  emd-34974.cif.gz emd-34974.cif.gz | 6.3 KB | ||
| Others |  emd_34974_half_map_1.map.gz emd_34974_half_map_1.map.gz emd_34974_half_map_2.map.gz emd_34974_half_map_2.map.gz | 475.7 MB 475.7 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-34974 http://ftp.pdbj.org/pub/emdb/structures/EMD-34974 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-34974 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-34974 | HTTPS FTP |
-Validation report
| Summary document |  emd_34974_validation.pdf.gz emd_34974_validation.pdf.gz | 847.6 KB | Display |  EMDB validaton report EMDB validaton report |
|---|---|---|---|---|
| Full document |  emd_34974_full_validation.pdf.gz emd_34974_full_validation.pdf.gz | 847.2 KB | Display | |
| Data in XML |  emd_34974_validation.xml.gz emd_34974_validation.xml.gz | 18.9 KB | Display | |
| Data in CIF |  emd_34974_validation.cif.gz emd_34974_validation.cif.gz | 22.6 KB | Display | |
| Arichive directory |  https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-34974 https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-34974 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-34974 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-34974 | HTTPS FTP |
-Related structure data
| Related structure data |  8hriMC  8hrjC  8hrkC  8hrlC  8hrmC  8hrnC  8hruC M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_34974.map.gz / Format: CCP4 / Size: 512 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_34974.map.gz / Format: CCP4 / Size: 512 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 0.86 Å | ||||||||||||||||||||||||||||||||||||
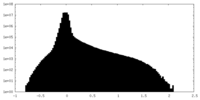
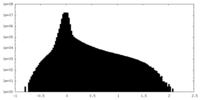
| Density |
| ||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
|
-Supplemental data
-Half map: #2
| File | emd_34974_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
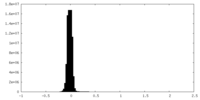
| Density Histograms |
-Half map: #1
| File | emd_34974_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
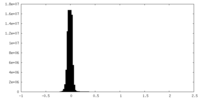
| Density Histograms |
- Sample components
Sample components
-Entire : SARS-CoV-2 Delta variant spike protein
| Entire | Name: SARS-CoV-2 Delta variant spike protein |
|---|---|
| Components |
|
-Supramolecule #1: SARS-CoV-2 Delta variant spike protein
| Supramolecule | Name: SARS-CoV-2 Delta variant spike protein / type: complex / ID: 1 / Parent: 0 / Macromolecule list: all / Details: on graphene membranes |
|---|---|
| Source (natural) | Organism:  |
-Macromolecule #1: Spike glycoprotein
| Macromolecule | Name: Spike glycoprotein / type: protein_or_peptide / ID: 1 Details: For issue of poor atom inclusion, the depositor stated 'we have tried our best to fit the coordinates in the density map of spike via rigid-body dock. However, the flexibility of RBD makes ...Details: For issue of poor atom inclusion, the depositor stated 'we have tried our best to fit the coordinates in the density map of spike via rigid-body dock. However, the flexibility of RBD makes it hard to fit, which seems to be general for spike proteins.' Number of copies: 3 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  |
| Molecular weight | Theoretical: 138.842375 KDa |
| Recombinant expression | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Sequence | String: MPRGPVAALL LLILHGAWSC VNLTTRTQLP PAYTNSFTRG VYYPDKVFRS SVLHSTQDLF LPFFSNVTWF HVISGTNGTK RFDNPVLPF NDGVYFASIE KSNIIRGWIF GTTLDSKTQS LLIVNNATNV VIKVCEFQFC NDPFLDHKNN KSWMESEFRV Y SSANNCTF ...String: MPRGPVAALL LLILHGAWSC VNLTTRTQLP PAYTNSFTRG VYYPDKVFRS SVLHSTQDLF LPFFSNVTWF HVISGTNGTK RFDNPVLPF NDGVYFASIE KSNIIRGWIF GTTLDSKTQS LLIVNNATNV VIKVCEFQFC NDPFLDHKNN KSWMESEFRV Y SSANNCTF EYVSQPFLMD LEGKQGNFKN LREFVFKNID GYFKIYSKHT PIIVREPEDL PQGFSALEPL VDLPIGINIT RF QTLLALH RSYLTPGDSS SGWTAGAAAY YVGYLQPRTF LLKYNENGTI TDAVDCALDP LSETKCTLKS FTVEKGIYQT SNF RVQPTE SIVRFPNITN LCPFDEVFNA TRFASVYAWN RKRISNCVAD YSVLYNLAPF FTFKCYGVSP TKLNDLCFTN VYAD SFVIR GDEVRQIAPG QTGNIADYNY KLPDDFTGCV IAWNSNKLDS KVSGNYNYLY RLFRKSNLKP FERDISTEIY QAGNK PCNG VAGFNCYFPL RSYSFRPTYG VGHQPYRVVV LSFELLHAPA TVCGPKKSTN LVKNKCVNFN FNGLKGTGVL TESNKK FLP FQQFGRDIAD TTDAVRDPQT LEILDITPCS FGGVSVITPG TNTSNQVAVL YQGVNCTEVP VAIHADQLTP TWRVYST GS NVFQTRAGCL IGAEYVNNSY ECDIPIGAGI CASYQTQTKS HGSASSVASQ SIIAYTMSLG AENSVAYSNN SIAIPTNF T ISVTTEILPV SMTKTSVDCT MYICGDSTEC SNLLLQYGSF CTQLKRALTG IAVEQDKNTQ EVFAQVKQIY KTPPIKYFG GFNFSQILPD PSKPSKRSFI EDLLFNKVTL ADAGFIKQYG DCLGDIAARD LICAQKFKGL TVLPPLLTDE MIAQYTSALL AGTITSGWT FGAGAALQIP FAMQMAYRFN GIGVTQNVLY ENQKLIANQF NSAIGKIQDS LSSTASALGK LQDVVNHNAQ A LNTLVKQL SSKFGAISSV LNDIFSRLDP PEAEVQIDRL ITGRLQSLQT YVTQQLIRAA EIRASANLAA TKMSECVLGQ SK RVDFCGK GYHLMSFPQS APHGVVFLHV TYVPAQEKNF TTAPAICHDG KAHFPREGVF VSNGTHWFVT QRNFYEPQII TTD NTFVSG NCDVVIGIVN NTVYDPLQPE LDSFKEELDK YFKNHTSPDV DLGDISGINA SVVNIQKEID RLNEVAKNLN ESLI DLQEL GKYEQLVPRG SGYIPEAPRD GQAYVRKDGE WVLLSTFLHH HHHH UniProtKB: Spike glycoprotein |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Concentration | 0.25 mg/mL |
|---|---|
| Buffer | pH: 7.8 |
| Vitrification | Cryogen name: ETHANE / Chamber humidity: 100 % / Chamber temperature: 281 K |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Image recording | Film or detector model: GATAN K3 BIOQUANTUM (6k x 4k) / Average electron dose: 50.0 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD / Nominal defocus max: 1.5 µm / Nominal defocus min: 1.2 µm |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
- Image processing
Image processing
| Startup model | Type of model: INSILICO MODEL |
|---|---|
| Final reconstruction | Resolution.type: BY AUTHOR / Resolution: 2.9 Å / Resolution method: FSC 0.143 CUT-OFF / Number images used: 167167 |
| Initial angle assignment | Type: MAXIMUM LIKELIHOOD |
| Final angle assignment | Type: MAXIMUM LIKELIHOOD |
 Movie
Movie Controller
Controller














 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)