+ データを開く
データを開く
- 基本情報
基本情報
| 登録情報 |  | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| タイトル | Cryo-EM structure of the galanin-bound GALR2-miniGq complex | |||||||||
 マップデータ マップデータ | ||||||||||
 試料 試料 |
| |||||||||
 キーワード キーワード | GPCR / galanin receptor 2 / mini-Gq / SIGNALING PROTEIN | |||||||||
| 機能・相同性 |  機能・相同性情報 機能・相同性情報galanin-activated signaling pathway / galanin receptor binding / type 1 galanin receptor binding / type 2 galanin receptor binding / type 3 galanin receptor binding / galanin receptor activity / positive regulation of large conductance calcium-activated potassium channel activity / parental behavior / positive regulation of timing of catagen / positive regulation of cortisol secretion ...galanin-activated signaling pathway / galanin receptor binding / type 1 galanin receptor binding / type 2 galanin receptor binding / type 3 galanin receptor binding / galanin receptor activity / positive regulation of large conductance calcium-activated potassium channel activity / parental behavior / positive regulation of timing of catagen / positive regulation of cortisol secretion / regulation of glucocorticoid metabolic process / negative regulation of lymphocyte proliferation / inositol phosphate metabolic process / phosphatidylinositol metabolic process / protein kinase A signaling / neuropeptide hormone activity / neuropeptide binding / feeding behavior / insulin secretion / response to immobilization stress / peptide hormone binding / neuropeptide signaling pathway / muscle contraction / secretory granule / Peptide ligand-binding receptors / response to insulin / adenylate cyclase-modulating G protein-coupled receptor signaling pathway / response to estrogen / Olfactory Signaling Pathway / Activation of the phototransduction cascade / G beta:gamma signalling through PLC beta / Presynaptic function of Kainate receptors / Thromboxane signalling through TP receptor / G protein-coupled acetylcholine receptor signaling pathway / G-protein activation / Activation of G protein gated Potassium channels / Inhibition of voltage gated Ca2+ channels via Gbeta/gamma subunits / adenylate cyclase-activating G protein-coupled receptor signaling pathway / Prostacyclin signalling through prostacyclin receptor / G beta:gamma signalling through CDC42 / Glucagon signaling in metabolic regulation / neuron projection development / G beta:gamma signalling through BTK / Synthesis, secretion, and inactivation of Glucagon-like Peptide-1 (GLP-1) / ADP signalling through P2Y purinoceptor 12 / Sensory perception of sweet, bitter, and umami (glutamate) taste / photoreceptor disc membrane / Glucagon-type ligand receptors / Adrenaline,noradrenaline inhibits insulin secretion / Vasopressin regulates renal water homeostasis via Aquaporins / Glucagon-like Peptide-1 (GLP1) regulates insulin secretion / G alpha (z) signalling events / ADP signalling through P2Y purinoceptor 1 / cellular response to catecholamine stimulus / ADORA2B mediated anti-inflammatory cytokines production / G beta:gamma signalling through PI3Kgamma / Cooperation of PDCL (PhLP1) and TRiC/CCT in G-protein beta folding / adenylate cyclase-activating dopamine receptor signaling pathway / GPER1 signaling / Inactivation, recovery and regulation of the phototransduction cascade / cellular response to prostaglandin E stimulus / G-protein beta-subunit binding / heterotrimeric G-protein complex / nervous system development / G alpha (12/13) signalling events / sensory perception of taste / extracellular vesicle / signaling receptor complex adaptor activity / Thrombin signalling through proteinase activated receptors (PARs) / retina development in camera-type eye / positive regulation of cytosolic calcium ion concentration / GTPase binding / Ca2+ pathway / fibroblast proliferation / High laminar flow shear stress activates signaling by PIEZO1 and PECAM1:CDH5:KDR in endothelial cells / G alpha (i) signalling events / G alpha (s) signalling events / phospholipase C-activating G protein-coupled receptor signaling pathway / G alpha (q) signalling events / Ras protein signal transduction / learning or memory / Extra-nuclear estrogen signaling / cell surface receptor signaling pathway / cell population proliferation / cilium / positive regulation of apoptotic process / G protein-coupled receptor signaling pathway / response to xenobiotic stimulus / lysosomal membrane / neuronal cell body / GTPase activity / synapse / protein-containing complex binding / signal transduction / positive regulation of transcription by RNA polymerase II / extracellular space / extracellular exosome / extracellular region / membrane / plasma membrane 類似検索 - 分子機能 | |||||||||
| 生物種 |  Homo sapiens (ヒト) Homo sapiens (ヒト) | |||||||||
| 手法 | 単粒子再構成法 / クライオ電子顕微鏡法 / 解像度: 3.3 Å | |||||||||
 データ登録者 データ登録者 | Jiang W / Zheng S | |||||||||
| 資金援助 |  中国, 1件 中国, 1件
| |||||||||
 引用 引用 |  ジャーナル: Proc Natl Acad Sci U S A / 年: 2022 ジャーナル: Proc Natl Acad Sci U S A / 年: 2022タイトル: Structural insights into galanin receptor signaling. 著者: Wentong Jiang / Sanduo Zheng /  要旨: Galanin is a biologically active neuropeptide, and functions through three distinct G protein–coupled receptors (GPCRs), namely GALR1, GALR2, and GALR3. GALR signaling plays important roles in ...Galanin is a biologically active neuropeptide, and functions through three distinct G protein–coupled receptors (GPCRs), namely GALR1, GALR2, and GALR3. GALR signaling plays important roles in regulating various physiological processes such as energy metabolism, neuropathic pain, epileptic activity, and sleep homeostasis. GALR1 and GALR3 signal through the Gi/o pathway, whereas GALR2 signals mainly through the Gq/11 pathway. However, the molecular basis for galanin recognition and G protein selectivity of GALRs remains poorly understood. Here, we report the cryoelectron microscopy structures of the GALR1-Go and the GALR2-Gq complexes bound to the endogenous ligand galanin or spexin. The galanin peptide mainly adopts an alpha helical structure, which binds at the extracellular vestibule of the receptors, nearly parallel to the membrane plane without penetrating deeply into the receptor core. Structural analysis combined with functional studies reveals important structural determinants for the G protein selectivity of GALRs as well as other class A GPCRs. In addition, we show that the zinc ion is a negative allosteric regulator of GALR1 but not GALR2. Our studies provide insight into the mechanisms of G protein selectivity of GPCRs and highlight a potential function of the neuromodulator zinc ion as a modulator of GPCR signaling in the central nervous system. | |||||||||
| 履歴 |
|
- 構造の表示
構造の表示
| 添付画像 |
|---|
- ダウンロードとリンク
ダウンロードとリンク
-EMDBアーカイブ
| マップデータ |  emd_33230.map.gz emd_33230.map.gz | 20.9 MB |  EMDBマップデータ形式 EMDBマップデータ形式 | |
|---|---|---|---|---|
| ヘッダ (付随情報) |  emd-33230-v30.xml emd-33230-v30.xml emd-33230.xml emd-33230.xml | 23.2 KB 23.2 KB | 表示 表示 |  EMDBヘッダ EMDBヘッダ |
| 画像 |  emd_33230.png emd_33230.png | 17 KB | ||
| Filedesc metadata |  emd-33230.cif.gz emd-33230.cif.gz | 6.9 KB | ||
| その他 |  emd_33230_half_map_1.map.gz emd_33230_half_map_1.map.gz emd_33230_half_map_2.map.gz emd_33230_half_map_2.map.gz | 20.6 MB 20.6 MB | ||
| アーカイブディレクトリ |  http://ftp.pdbj.org/pub/emdb/structures/EMD-33230 http://ftp.pdbj.org/pub/emdb/structures/EMD-33230 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-33230 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-33230 | HTTPS FTP |
-検証レポート
| 文書・要旨 |  emd_33230_validation.pdf.gz emd_33230_validation.pdf.gz | 775.5 KB | 表示 |  EMDB検証レポート EMDB検証レポート |
|---|---|---|---|---|
| 文書・詳細版 |  emd_33230_full_validation.pdf.gz emd_33230_full_validation.pdf.gz | 775.1 KB | 表示 | |
| XML形式データ |  emd_33230_validation.xml.gz emd_33230_validation.xml.gz | 10.3 KB | 表示 | |
| CIF形式データ |  emd_33230_validation.cif.gz emd_33230_validation.cif.gz | 12 KB | 表示 | |
| アーカイブディレクトリ |  https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-33230 https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-33230 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-33230 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-33230 | HTTPS FTP |
-関連構造データ
- リンク
リンク
| EMDBのページ |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| 「今月の分子」の関連する項目 |
- マップ
マップ
| ファイル |  ダウンロード / ファイル: emd_33230.map.gz / 形式: CCP4 / 大きさ: 22.2 MB / タイプ: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) ダウンロード / ファイル: emd_33230.map.gz / 形式: CCP4 / 大きさ: 22.2 MB / タイプ: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 投影像・断面図 | 画像のコントロール
画像は Spider により作成 | ||||||||||||||||||||||||||||||||||||
| ボクセルのサイズ | X=Y=Z: 1.087 Å | ||||||||||||||||||||||||||||||||||||
| 密度 |
| ||||||||||||||||||||||||||||||||||||
| 対称性 | 空間群: 1 | ||||||||||||||||||||||||||||||||||||
| 詳細 | EMDB XML:
|
-添付データ
-ハーフマップ: #1
| ファイル | emd_33230_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
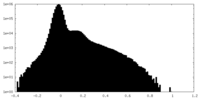
| 投影像・断面図 |
| ||||||||||||
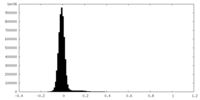
| 密度ヒストグラム |
-ハーフマップ: #2
| ファイル | emd_33230_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
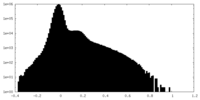
| 投影像・断面図 |
| ||||||||||||
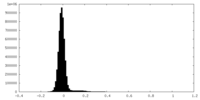
| 密度ヒストグラム |
- 試料の構成要素
試料の構成要素
-全体 : Galanin-bound Galanin receptor 2 in complex with Galphaq, Gbeta/g...
| 全体 | 名称: Galanin-bound Galanin receptor 2 in complex with Galphaq, Gbeta/gamma subunit and single chain variable fragment (scFv16) |
|---|---|
| 要素 |
|
-超分子 #1: Galanin-bound Galanin receptor 2 in complex with Galphaq, Gbeta/g...
| 超分子 | 名称: Galanin-bound Galanin receptor 2 in complex with Galphaq, Gbeta/gamma subunit and single chain variable fragment (scFv16) タイプ: complex / ID: 1 / 親要素: 0 / 含まれる分子: all |
|---|---|
| 由来(天然) | 生物種:  Homo sapiens (ヒト) Homo sapiens (ヒト) |
-分子 #1: Galanin
| 分子 | 名称: Galanin / タイプ: protein_or_peptide / ID: 1 / コピー数: 1 / 光学異性体: LEVO |
|---|---|
| 由来(天然) | 生物種:  Homo sapiens (ヒト) Homo sapiens (ヒト) |
| 分子量 | 理論値: 3.161446 KDa |
| 組換発現 | 生物種: synthetic construct (人工物) |
| 配列 | 文字列: GWTLNSAGYL LGPHAVGNHR SFSDKNGLTS UniProtKB: Galanin peptides |
-分子 #2: Guanine nucleotide-binding protein G(q)
| 分子 | 名称: Guanine nucleotide-binding protein G(q) / タイプ: protein_or_peptide / ID: 2 / コピー数: 1 / 光学異性体: LEVO |
|---|---|
| 由来(天然) | 生物種:  Homo sapiens (ヒト) Homo sapiens (ヒト) |
| 分子量 | 理論値: 28.026797 KDa |
| 組換発現 | 生物種:  Homo sapiens (ヒト) Homo sapiens (ヒト) |
| 配列 | 文字列: MGSTVSAEDK AAAERSKMID KNLREDGEKA RRTLRLLLLG ADNSGKSTIV KQMRILHGGS GGSGGTSGIF ETKFQVDKVN FHMFDVGGQ RDERRKWIQC FNDVTAIIFV VDSSDYNRLQ EALNDFKSIW NNRWLRTISV ILFLNKQDLL AEKVLAGKSK I EDYFPEFA ...文字列: MGSTVSAEDK AAAERSKMID KNLREDGEKA RRTLRLLLLG ADNSGKSTIV KQMRILHGGS GGSGGTSGIF ETKFQVDKVN FHMFDVGGQ RDERRKWIQC FNDVTAIIFV VDSSDYNRLQ EALNDFKSIW NNRWLRTISV ILFLNKQDLL AEKVLAGKSK I EDYFPEFA RYTTPEAATP EPGDDPRVTR AKYFIRKEFV DISTASGDGR HICYPHFTCA VDTENARRIF NDCKDIILQM NL REYNLV |
-分子 #3: Guanine nucleotide-binding protein G(I)/G(S)/G(T) subunit beta-1
| 分子 | 名称: Guanine nucleotide-binding protein G(I)/G(S)/G(T) subunit beta-1 タイプ: protein_or_peptide / ID: 3 / コピー数: 1 / 光学異性体: LEVO |
|---|---|
| 由来(天然) | 生物種:  Homo sapiens (ヒト) Homo sapiens (ヒト) |
| 分子量 | 理論値: 38.389934 KDa |
| 組換発現 | 生物種:  |
| 配列 | 文字列: HHHHHHGSMS ELDQLRQEAE QLKNQIRDAR KACADATLSQ ITNNIDPVGR IQMRTRRTLR GHLAKIYAMH WGTDSRLLVS ASQDGKLII WDSYTTNKVH AIPLRSSWVM TCAYAPSGNY VACGGLDNIC SIYNLKTREG NVRVSRELAG HTGYLSCCRF L DDNQIVTS ...文字列: HHHHHHGSMS ELDQLRQEAE QLKNQIRDAR KACADATLSQ ITNNIDPVGR IQMRTRRTLR GHLAKIYAMH WGTDSRLLVS ASQDGKLII WDSYTTNKVH AIPLRSSWVM TCAYAPSGNY VACGGLDNIC SIYNLKTREG NVRVSRELAG HTGYLSCCRF L DDNQIVTS SGDTTCALWD IETGQQTTTF TGHTGDVMSL SLAPDTRLFV SGACDASAKL WDVREGMCRQ TFTGHESDIN AI CFFPNGN AFATGSDDAT CRLFDLRADQ ELMTYSHDNI ICGITSVSFS KSGRLLLAGY DDFNCNVWDA LKADRAGVLA GHD NRVSCL GVTDDGMAVA TGSWDSFLKI WN UniProtKB: Guanine nucleotide-binding protein G(I)/G(S)/G(T) subunit beta-1 |
-分子 #4: Guanine nucleotide-binding protein G(I)/G(S)/G(O) subunit gamma-2
| 分子 | 名称: Guanine nucleotide-binding protein G(I)/G(S)/G(O) subunit gamma-2 タイプ: protein_or_peptide / ID: 4 / コピー数: 1 / 光学異性体: LEVO |
|---|---|
| 由来(天然) | 生物種:  Homo sapiens (ヒト) Homo sapiens (ヒト) |
| 分子量 | 理論値: 7.845078 KDa |
| 組換発現 | 生物種:  |
| 配列 | 文字列: MASNNTASIA QARKLVEQLK MEANIDRIKV SKAAADLMAY CEAHAKEDPL LTPVPASENP FREKKFFSAI L UniProtKB: Guanine nucleotide-binding protein G(I)/G(S)/G(O) subunit gamma-2 |
-分子 #5: single Fab chain (svFv16)
| 分子 | 名称: single Fab chain (svFv16) / タイプ: protein_or_peptide / ID: 5 / コピー数: 1 / 光学異性体: LEVO |
|---|---|
| 由来(天然) | 生物種:  Homo sapiens (ヒト) Homo sapiens (ヒト) |
| 分子量 | 理論値: 32.071615 KDa |
| 組換発現 | 生物種:  |
| 配列 | 文字列: GRPDVQLVES GGGLVQPGGS RKLSCSASGF AFSSFGMHWV RQAPEKGLEW VAYISSGSGT IYYADTVKGR FTISRDDPKN TLFLQMTSL RSEDTAMYYC VRSIYYYGSS PFDFWGQGTT LTVSSGGGGS GGGGSGGGGS DIVMTQATSS VPVTPGESVS I SCRSSKSL ...文字列: GRPDVQLVES GGGLVQPGGS RKLSCSASGF AFSSFGMHWV RQAPEKGLEW VAYISSGSGT IYYADTVKGR FTISRDDPKN TLFLQMTSL RSEDTAMYYC VRSIYYYGSS PFDFWGQGTT LTVSSGGGGS GGGGSGGGGS DIVMTQATSS VPVTPGESVS I SCRSSKSL LHSNGNTYLY WFLQRPGQSP QLLIYRMSNL ASGVPDRFSG SGSGTAFTLT ISRLEAEDVG VYYCMQHLEY PL TFGAGTK LELKAAAGAP LEVLFQGPGA WSHPQFEKGA EDQVDPRLID GKGAAHHHHH HHH |
-分子 #6: Galanin receptor type 2
| 分子 | 名称: Galanin receptor type 2 / タイプ: protein_or_peptide / ID: 6 / コピー数: 1 / 光学異性体: LEVO |
|---|---|
| 由来(天然) | 生物種:  Homo sapiens (ヒト) Homo sapiens (ヒト) |
| 分子量 | 理論値: 36.401867 KDa |
| 組換発現 | 生物種:  Homo sapiens (ヒト) Homo sapiens (ヒト) |
| 配列 | 文字列: DYKDDDDKGS MNVSGCPGAG NASQAGGGGG WHPEAVIVPL LFALIFLVGT VGNTLVLAVL LRGGQAVSTT NLFILNLGVA DLCFILCCV PFQATIYTLD GWVFGSLLCK AVHFLIFLTM HASSFTLAAV SLDRYLAIRY PLHSRELRTP RNALAAIGLI W GLSLLFSG ...文字列: DYKDDDDKGS MNVSGCPGAG NASQAGGGGG WHPEAVIVPL LFALIFLVGT VGNTLVLAVL LRGGQAVSTT NLFILNLGVA DLCFILCCV PFQATIYTLD GWVFGSLLCK AVHFLIFLTM HASSFTLAAV SLDRYLAIRY PLHSRELRTP RNALAAIGLI W GLSLLFSG PYLSYYQQSQ LANLTVCHPA WSAPRRRAMD ICTFVFSYLL PVLVLGLTYA RTLRYLWRAV DPVAAGSGAR RA KRKVTRM ILIVAALFCL CWMPHHALIL CVWFGQFPLT RATYALRILS HLVSYANSCV NPIVYALVSK HFRKGFRTIC AGL LGRAGS LEVLFQ UniProtKB: Galanin receptor type 2 |
-実験情報
-構造解析
| 手法 | クライオ電子顕微鏡法 |
|---|---|
 解析 解析 | 単粒子再構成法 |
| 試料の集合状態 | 3D array |
- 試料調製
試料調製
| 濃度 | 5.6 mg/mL | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 緩衝液 | pH: 7.4 構成要素:
| ||||||||||
| グリッド | モデル: Quantifoil R1.2/1.3 / 材質: GOLD / 支持フィルム - 材質: CARBON / 支持フィルム - トポロジー: HOLEY / 前処理 - タイプ: GLOW DISCHARGE / 前処理 - 時間: 45 sec. / 前処理 - 雰囲気: AIR / 前処理 - 気圧: 101.325 kPa | ||||||||||
| 凍結 | 凍結剤: ETHANE |
- 電子顕微鏡法
電子顕微鏡法
| 顕微鏡 | FEI TITAN KRIOS |
|---|---|
| 撮影 | フィルム・検出器のモデル: GATAN K3 BIOQUANTUM (6k x 4k) 平均電子線量: 50.0 e/Å2 |
| 電子線 | 加速電圧: 300 kV / 電子線源:  FIELD EMISSION GUN FIELD EMISSION GUN |
| 電子光学系 | 照射モード: FLOOD BEAM / 撮影モード: BRIGHT FIELD 最大 デフォーカス(公称値): 2.3000000000000003 µm 最小 デフォーカス(公称値): 1.2 µm |
| 試料ステージ | ホルダー冷却材: NITROGEN |
| 実験機器 |  モデル: Titan Krios / 画像提供: FEI Company |
+ 画像解析
画像解析
-原子モデル構築 1
| 精密化 | 空間: REAL / プロトコル: RIGID BODY FIT |
|---|---|
| 得られたモデル |  PDB-7xjk: |
 ムービー
ムービー コントローラー
コントローラー































 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)