+ Open data
Open data
- Basic information
Basic information
| Entry |  | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | Cryo-EM structure of the galanin-bound GALR2-miniGq complex | |||||||||
 Map data Map data | ||||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | GPCR / galanin receptor 2 / mini-Gq / SIGNALING PROTEIN | |||||||||
| Function / homology |  Function and homology information Function and homology informationgalanin-activated signaling pathway / galanin receptor binding / type 1 galanin receptor binding / type 2 galanin receptor binding / type 3 galanin receptor binding / positive regulation of large conductance calcium-activated potassium channel activity / parental behavior / galanin receptor activity / positive regulation of timing of catagen / positive regulation of cortisol secretion ...galanin-activated signaling pathway / galanin receptor binding / type 1 galanin receptor binding / type 2 galanin receptor binding / type 3 galanin receptor binding / positive regulation of large conductance calcium-activated potassium channel activity / parental behavior / galanin receptor activity / positive regulation of timing of catagen / positive regulation of cortisol secretion / regulation of glucocorticoid metabolic process / inositol phosphate metabolic process / negative regulation of lymphocyte proliferation / phosphatidylinositol metabolic process / : / neuropeptide hormone activity / feeding behavior / neuropeptide binding / insulin secretion / response to immobilization stress / peptide hormone binding / neuropeptide signaling pathway / muscle contraction / secretory granule / Peptide ligand-binding receptors / response to insulin / response to estrogen / adenylate cyclase-modulating G protein-coupled receptor signaling pathway / Olfactory Signaling Pathway / adenylate cyclase-activating G protein-coupled receptor signaling pathway / neuron projection development / Activation of the phototransduction cascade / G beta:gamma signalling through PLC beta / Presynaptic function of Kainate receptors / Thromboxane signalling through TP receptor / G protein-coupled acetylcholine receptor signaling pathway / Activation of G protein gated Potassium channels / Inhibition of voltage gated Ca2+ channels via Gbeta/gamma subunits / G-protein activation / G beta:gamma signalling through CDC42 / Prostacyclin signalling through prostacyclin receptor / Glucagon signaling in metabolic regulation / G beta:gamma signalling through BTK / Synthesis, secretion, and inactivation of Glucagon-like Peptide-1 (GLP-1) / ADP signalling through P2Y purinoceptor 12 / photoreceptor disc membrane / Glucagon-type ligand receptors / Sensory perception of sweet, bitter, and umami (glutamate) taste / Adrenaline,noradrenaline inhibits insulin secretion / Vasopressin regulates renal water homeostasis via Aquaporins / Glucagon-like Peptide-1 (GLP1) regulates insulin secretion / G alpha (z) signalling events / ADP signalling through P2Y purinoceptor 1 / cellular response to catecholamine stimulus / ADORA2B mediated anti-inflammatory cytokines production / G beta:gamma signalling through PI3Kgamma / adenylate cyclase-activating dopamine receptor signaling pathway / Cooperation of PDCL (PhLP1) and TRiC/CCT in G-protein beta folding / nervous system development / GPER1 signaling / G-protein beta-subunit binding / cellular response to prostaglandin E stimulus / heterotrimeric G-protein complex / G alpha (12/13) signalling events / Inactivation, recovery and regulation of the phototransduction cascade / extracellular vesicle / sensory perception of taste / Thrombin signalling through proteinase activated receptors (PARs) / signaling receptor complex adaptor activity / retina development in camera-type eye / positive regulation of cytosolic calcium ion concentration / GTPase binding / Ca2+ pathway / fibroblast proliferation / High laminar flow shear stress activates signaling by PIEZO1 and PECAM1:CDH5:KDR in endothelial cells / G alpha (i) signalling events / G alpha (s) signalling events / phospholipase C-activating G protein-coupled receptor signaling pathway / G alpha (q) signalling events / Ras protein signal transduction / learning or memory / cell surface receptor signaling pathway / Extra-nuclear estrogen signaling / cell population proliferation / cilium / positive regulation of apoptotic process / G protein-coupled receptor signaling pathway / response to xenobiotic stimulus / lysosomal membrane / neuronal cell body / GTPase activity / synapse / protein-containing complex binding / signal transduction / positive regulation of transcription by RNA polymerase II / extracellular space / extracellular exosome / extracellular region / membrane / plasma membrane Similarity search - Function | |||||||||
| Biological species |  Homo sapiens (human) Homo sapiens (human) | |||||||||
| Method | single particle reconstruction / cryo EM / Resolution: 3.3 Å | |||||||||
 Authors Authors | Jiang W / Zheng S | |||||||||
| Funding support |  China, 1 items China, 1 items
| |||||||||
 Citation Citation |  Journal: Proc Natl Acad Sci U S A / Year: 2022 Journal: Proc Natl Acad Sci U S A / Year: 2022Title: Structural insights into galanin receptor signaling. Authors: Wentong Jiang / Sanduo Zheng /  Abstract: Galanin is a biologically active neuropeptide, and functions through three distinct G protein–coupled receptors (GPCRs), namely GALR1, GALR2, and GALR3. GALR signaling plays important roles in ...Galanin is a biologically active neuropeptide, and functions through three distinct G protein–coupled receptors (GPCRs), namely GALR1, GALR2, and GALR3. GALR signaling plays important roles in regulating various physiological processes such as energy metabolism, neuropathic pain, epileptic activity, and sleep homeostasis. GALR1 and GALR3 signal through the Gi/o pathway, whereas GALR2 signals mainly through the Gq/11 pathway. However, the molecular basis for galanin recognition and G protein selectivity of GALRs remains poorly understood. Here, we report the cryoelectron microscopy structures of the GALR1-Go and the GALR2-Gq complexes bound to the endogenous ligand galanin or spexin. The galanin peptide mainly adopts an alpha helical structure, which binds at the extracellular vestibule of the receptors, nearly parallel to the membrane plane without penetrating deeply into the receptor core. Structural analysis combined with functional studies reveals important structural determinants for the G protein selectivity of GALRs as well as other class A GPCRs. In addition, we show that the zinc ion is a negative allosteric regulator of GALR1 but not GALR2. Our studies provide insight into the mechanisms of G protein selectivity of GPCRs and highlight a potential function of the neuromodulator zinc ion as a modulator of GPCR signaling in the central nervous system. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Supplemental images |
|---|
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_33230.map.gz emd_33230.map.gz | 20.9 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-33230-v30.xml emd-33230-v30.xml emd-33230.xml emd-33230.xml | 23.2 KB 23.2 KB | Display Display |  EMDB header EMDB header |
| Images |  emd_33230.png emd_33230.png | 17 KB | ||
| Filedesc metadata |  emd-33230.cif.gz emd-33230.cif.gz | 6.9 KB | ||
| Others |  emd_33230_half_map_1.map.gz emd_33230_half_map_1.map.gz emd_33230_half_map_2.map.gz emd_33230_half_map_2.map.gz | 20.6 MB 20.6 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-33230 http://ftp.pdbj.org/pub/emdb/structures/EMD-33230 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-33230 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-33230 | HTTPS FTP |
-Related structure data
| Related structure data |  7xjkMC  7xjjC  7xjlC M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_33230.map.gz / Format: CCP4 / Size: 22.2 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_33230.map.gz / Format: CCP4 / Size: 22.2 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 1.087 Å | ||||||||||||||||||||||||||||||||||||
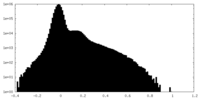
| Density |
| ||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
|
-Supplemental data
-Half map: #1
| File | emd_33230_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
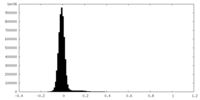
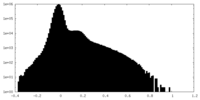
| Density Histograms |
-Half map: #2
| File | emd_33230_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
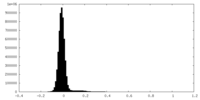
| Density Histograms |
- Sample components
Sample components
-Entire : Galanin-bound Galanin receptor 2 in complex with Galphaq, Gbeta/g...
| Entire | Name: Galanin-bound Galanin receptor 2 in complex with Galphaq, Gbeta/gamma subunit and single chain variable fragment (scFv16) |
|---|---|
| Components |
|
-Supramolecule #1: Galanin-bound Galanin receptor 2 in complex with Galphaq, Gbeta/g...
| Supramolecule | Name: Galanin-bound Galanin receptor 2 in complex with Galphaq, Gbeta/gamma subunit and single chain variable fragment (scFv16) type: complex / ID: 1 / Parent: 0 / Macromolecule list: all |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
-Macromolecule #1: Galanin
| Macromolecule | Name: Galanin / type: protein_or_peptide / ID: 1 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 3.161446 KDa |
| Recombinant expression | Organism: synthetic construct (others) |
| Sequence | String: GWTLNSAGYL LGPHAVGNHR SFSDKNGLTS UniProtKB: Galanin peptides |
-Macromolecule #2: Guanine nucleotide-binding protein G(q)
| Macromolecule | Name: Guanine nucleotide-binding protein G(q) / type: protein_or_peptide / ID: 2 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 28.026797 KDa |
| Recombinant expression | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Sequence | String: MGSTVSAEDK AAAERSKMID KNLREDGEKA RRTLRLLLLG ADNSGKSTIV KQMRILHGGS GGSGGTSGIF ETKFQVDKVN FHMFDVGGQ RDERRKWIQC FNDVTAIIFV VDSSDYNRLQ EALNDFKSIW NNRWLRTISV ILFLNKQDLL AEKVLAGKSK I EDYFPEFA ...String: MGSTVSAEDK AAAERSKMID KNLREDGEKA RRTLRLLLLG ADNSGKSTIV KQMRILHGGS GGSGGTSGIF ETKFQVDKVN FHMFDVGGQ RDERRKWIQC FNDVTAIIFV VDSSDYNRLQ EALNDFKSIW NNRWLRTISV ILFLNKQDLL AEKVLAGKSK I EDYFPEFA RYTTPEAATP EPGDDPRVTR AKYFIRKEFV DISTASGDGR HICYPHFTCA VDTENARRIF NDCKDIILQM NL REYNLV |
-Macromolecule #3: Guanine nucleotide-binding protein G(I)/G(S)/G(T) subunit beta-1
| Macromolecule | Name: Guanine nucleotide-binding protein G(I)/G(S)/G(T) subunit beta-1 type: protein_or_peptide / ID: 3 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 38.389934 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: HHHHHHGSMS ELDQLRQEAE QLKNQIRDAR KACADATLSQ ITNNIDPVGR IQMRTRRTLR GHLAKIYAMH WGTDSRLLVS ASQDGKLII WDSYTTNKVH AIPLRSSWVM TCAYAPSGNY VACGGLDNIC SIYNLKTREG NVRVSRELAG HTGYLSCCRF L DDNQIVTS ...String: HHHHHHGSMS ELDQLRQEAE QLKNQIRDAR KACADATLSQ ITNNIDPVGR IQMRTRRTLR GHLAKIYAMH WGTDSRLLVS ASQDGKLII WDSYTTNKVH AIPLRSSWVM TCAYAPSGNY VACGGLDNIC SIYNLKTREG NVRVSRELAG HTGYLSCCRF L DDNQIVTS SGDTTCALWD IETGQQTTTF TGHTGDVMSL SLAPDTRLFV SGACDASAKL WDVREGMCRQ TFTGHESDIN AI CFFPNGN AFATGSDDAT CRLFDLRADQ ELMTYSHDNI ICGITSVSFS KSGRLLLAGY DDFNCNVWDA LKADRAGVLA GHD NRVSCL GVTDDGMAVA TGSWDSFLKI WN UniProtKB: Guanine nucleotide-binding protein G(I)/G(S)/G(T) subunit beta-1 |
-Macromolecule #4: Guanine nucleotide-binding protein G(I)/G(S)/G(O) subunit gamma-2
| Macromolecule | Name: Guanine nucleotide-binding protein G(I)/G(S)/G(O) subunit gamma-2 type: protein_or_peptide / ID: 4 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 7.845078 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: MASNNTASIA QARKLVEQLK MEANIDRIKV SKAAADLMAY CEAHAKEDPL LTPVPASENP FREKKFFSAI L UniProtKB: Guanine nucleotide-binding protein G(I)/G(S)/G(O) subunit gamma-2 |
-Macromolecule #5: single Fab chain (svFv16)
| Macromolecule | Name: single Fab chain (svFv16) / type: protein_or_peptide / ID: 5 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 32.071615 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: GRPDVQLVES GGGLVQPGGS RKLSCSASGF AFSSFGMHWV RQAPEKGLEW VAYISSGSGT IYYADTVKGR FTISRDDPKN TLFLQMTSL RSEDTAMYYC VRSIYYYGSS PFDFWGQGTT LTVSSGGGGS GGGGSGGGGS DIVMTQATSS VPVTPGESVS I SCRSSKSL ...String: GRPDVQLVES GGGLVQPGGS RKLSCSASGF AFSSFGMHWV RQAPEKGLEW VAYISSGSGT IYYADTVKGR FTISRDDPKN TLFLQMTSL RSEDTAMYYC VRSIYYYGSS PFDFWGQGTT LTVSSGGGGS GGGGSGGGGS DIVMTQATSS VPVTPGESVS I SCRSSKSL LHSNGNTYLY WFLQRPGQSP QLLIYRMSNL ASGVPDRFSG SGSGTAFTLT ISRLEAEDVG VYYCMQHLEY PL TFGAGTK LELKAAAGAP LEVLFQGPGA WSHPQFEKGA EDQVDPRLID GKGAAHHHHH HHH |
-Macromolecule #6: Galanin receptor type 2
| Macromolecule | Name: Galanin receptor type 2 / type: protein_or_peptide / ID: 6 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 36.401867 KDa |
| Recombinant expression | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Sequence | String: DYKDDDDKGS MNVSGCPGAG NASQAGGGGG WHPEAVIVPL LFALIFLVGT VGNTLVLAVL LRGGQAVSTT NLFILNLGVA DLCFILCCV PFQATIYTLD GWVFGSLLCK AVHFLIFLTM HASSFTLAAV SLDRYLAIRY PLHSRELRTP RNALAAIGLI W GLSLLFSG ...String: DYKDDDDKGS MNVSGCPGAG NASQAGGGGG WHPEAVIVPL LFALIFLVGT VGNTLVLAVL LRGGQAVSTT NLFILNLGVA DLCFILCCV PFQATIYTLD GWVFGSLLCK AVHFLIFLTM HASSFTLAAV SLDRYLAIRY PLHSRELRTP RNALAAIGLI W GLSLLFSG PYLSYYQQSQ LANLTVCHPA WSAPRRRAMD ICTFVFSYLL PVLVLGLTYA RTLRYLWRAV DPVAAGSGAR RA KRKVTRM ILIVAALFCL CWMPHHALIL CVWFGQFPLT RATYALRILS HLVSYANSCV NPIVYALVSK HFRKGFRTIC AGL LGRAGS LEVLFQ UniProtKB: Galanin receptor type 2 |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | 3D array |
- Sample preparation
Sample preparation
| Concentration | 5.6 mg/mL | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Buffer | pH: 7.4 Component:
| ||||||||||
| Grid | Model: Quantifoil R1.2/1.3 / Material: GOLD / Support film - Material: CARBON / Support film - topology: HOLEY / Pretreatment - Type: GLOW DISCHARGE / Pretreatment - Time: 45 sec. / Pretreatment - Atmosphere: AIR / Pretreatment - Pressure: 101.325 kPa | ||||||||||
| Vitrification | Cryogen name: ETHANE |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Image recording | Film or detector model: GATAN K3 BIOQUANTUM (6k x 4k) / Average electron dose: 50.0 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD / Nominal defocus max: 2.3000000000000003 µm / Nominal defocus min: 1.2 µm |
| Sample stage | Cooling holder cryogen: NITROGEN |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
+ Image processing
Image processing
-Atomic model buiding 1
| Refinement | Space: REAL / Protocol: RIGID BODY FIT |
|---|---|
| Output model |  PDB-7xjk: |
 Movie
Movie Controller
Controller





























 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)