+ Open data
Open data
- Basic information
Basic information
| Entry |  | ||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Title | human RAD51 presynaptic complex | ||||||||||||||||||||||||
 Map data Map data | cryoEM structure of human RAD51 presynaptic complex. | ||||||||||||||||||||||||
 Sample Sample |
| ||||||||||||||||||||||||
 Keywords Keywords | DNA BINDING PROTEIN-DNA complex | ||||||||||||||||||||||||
| Function / homology |  Function and homology information Function and homology informationpresynaptic intermediate filament cytoskeleton / response to glucoside / mitotic recombination-dependent replication fork processing / DNA recombinase assembly / cellular response to camptothecin / chromosome organization involved in meiotic cell cycle / telomere maintenance via telomere lengthening / double-strand break repair involved in meiotic recombination / nuclear ubiquitin ligase complex / cellular response to cisplatin ...presynaptic intermediate filament cytoskeleton / response to glucoside / mitotic recombination-dependent replication fork processing / DNA recombinase assembly / cellular response to camptothecin / chromosome organization involved in meiotic cell cycle / telomere maintenance via telomere lengthening / double-strand break repair involved in meiotic recombination / nuclear ubiquitin ligase complex / cellular response to cisplatin / DNA strand invasion / cellular response to hydroxyurea / mitotic recombination / DNA strand exchange activity / replication-born double-strand break repair via sister chromatid exchange / lateral element / regulation of DNA damage checkpoint / Impaired BRCA2 binding to PALB2 / telomere maintenance via recombination / single-stranded DNA helicase activity / reciprocal meiotic recombination / Homologous DNA Pairing and Strand Exchange / Defective homologous recombination repair (HRR) due to BRCA1 loss of function / Defective HDR through Homologous Recombination Repair (HRR) due to PALB2 loss of BRCA1 binding function / Defective HDR through Homologous Recombination Repair (HRR) due to PALB2 loss of BRCA2/RAD51/RAD51C binding function / Resolution of D-loop Structures through Synthesis-Dependent Strand Annealing (SDSA) / Resolution of D-loop Structures through Holliday Junction Intermediates / HDR through Single Strand Annealing (SSA) / ATP-dependent DNA damage sensor activity / regulation of double-strand break repair via homologous recombination / nuclear chromosome / Impaired BRCA2 binding to RAD51 / Transcriptional Regulation by E2F6 / replication fork processing / Presynaptic phase of homologous DNA pairing and strand exchange / response to X-ray / ATP-dependent activity, acting on DNA / interstrand cross-link repair / condensed chromosome / DNA polymerase binding / condensed nuclear chromosome / cellular response to ionizing radiation / male germ cell nucleus / meiotic cell cycle / cellular response to gamma radiation / double-strand break repair via homologous recombination / PML body / HDR through Homologous Recombination (HRR) / Meiotic recombination / response to toxic substance / single-stranded DNA binding / site of double-strand break / double-stranded DNA binding / DNA recombination / chromosome, telomeric region / mitochondrial matrix / response to xenobiotic stimulus / DNA repair / DNA damage response / chromatin binding / centrosome / chromatin / nucleolus / perinuclear region of cytoplasm / enzyme binding / ATP hydrolysis activity / protein-containing complex / mitochondrion / nucleoplasm / ATP binding / identical protein binding / nucleus / cytosol / cytoplasm Similarity search - Function | ||||||||||||||||||||||||
| Biological species |  Homo sapiens (human) Homo sapiens (human) | ||||||||||||||||||||||||
| Method | helical reconstruction / cryo EM / Resolution: 2.97 Å | ||||||||||||||||||||||||
 Authors Authors | Zhao LY / Xu JF / Wang HW | ||||||||||||||||||||||||
| Funding support |  China, 7 items China, 7 items
| ||||||||||||||||||||||||
 Citation Citation |  Journal: Nucleic Acids Res / Year: 2021 Journal: Nucleic Acids Res / Year: 2021Title: Mechanisms of distinctive mismatch tolerance between Rad51 and Dmc1 in homologous recombination. Authors: Jingfei Xu / Lingyun Zhao / Sijia Peng / Huiying Chu / Rui Liang / Meng Tian / Philip P Connell / Guohui Li / Chunlai Chen / Hong-Wei Wang /    Abstract: Homologous recombination (HR) is a primary DNA double-strand breaks (DSBs) repair mechanism. The recombinases Rad51 and Dmc1 are highly conserved in the RecA family; Rad51 is mainly responsible for ...Homologous recombination (HR) is a primary DNA double-strand breaks (DSBs) repair mechanism. The recombinases Rad51 and Dmc1 are highly conserved in the RecA family; Rad51 is mainly responsible for DNA repair in somatic cells during mitosis while Dmc1 only works during meiosis in germ cells. This spatiotemporal difference is probably due to their distinctive mismatch tolerance during HR: Rad51 does not permit HR in the presence of mismatches, whereas Dmc1 can tolerate certain mismatches. Here, the cryo-EM structures of Rad51-DNA and Dmc1-DNA complexes revealed that the major conformational differences between these two proteins are located in their Loop2 regions, which contain invading single-stranded DNA (ssDNA) binding residues and double-stranded DNA (dsDNA) complementary strand binding residues, stabilizing ssDNA and dsDNA in presynaptic and postsynaptic complexes, respectively. By combining molecular dynamic simulation and single-molecule FRET assays, we identified that V273 and D274 in the Loop2 region of human RAD51 (hRAD51), corresponding to P274 and G275 of human DMC1 (hDMC1), are the key residues regulating mismatch tolerance during strand exchange in HR. This HR accuracy control mechanism provides mechanistic insights into the specific roles of Rad51 and Dmc1 in DNA double-strand break repair and may shed light on the regulatory mechanism of genetic recombination in mitosis and meiosis. | ||||||||||||||||||||||||
| History |
|
- Structure visualization
Structure visualization
| Supplemental images |
|---|
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_31158.map.gz emd_31158.map.gz | 21.8 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-31158-v30.xml emd-31158-v30.xml emd-31158.xml emd-31158.xml | 13.9 KB 13.9 KB | Display Display |  EMDB header EMDB header |
| Images |  emd_31158.png emd_31158.png | 273.5 KB | ||
| Filedesc metadata |  emd-31158.cif.gz emd-31158.cif.gz | 5.8 KB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-31158 http://ftp.pdbj.org/pub/emdb/structures/EMD-31158 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-31158 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-31158 | HTTPS FTP |
-Related structure data
| Related structure data |  7ejcMC  7ej6C  7ej7C  7ejeC M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_31158.map.gz / Format: CCP4 / Size: 244.1 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_31158.map.gz / Format: CCP4 / Size: 244.1 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | cryoEM structure of human RAD51 presynaptic complex. | ||||||||||||||||||||||||||||||||||||
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 0.885 Å | ||||||||||||||||||||||||||||||||||||
| Density |
| ||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
|
-Supplemental data
- Sample components
Sample components
-Entire : Human RAD51 presynaptic complex
| Entire | Name: Human RAD51 presynaptic complex |
|---|---|
| Components |
|
-Supramolecule #1: Human RAD51 presynaptic complex
| Supramolecule | Name: Human RAD51 presynaptic complex / type: complex / ID: 1 / Parent: 0 / Macromolecule list: #1-#2 Details: 2.97 angstrom cryoEM structure of human RAD51 presynaptic complex |
|---|
-Supramolecule #2: DNA
| Supramolecule | Name: DNA / type: complex / ID: 2 / Parent: 1 / Macromolecule list: #1 |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) / Synthetically produced: Yes Homo sapiens (human) / Synthetically produced: Yes |
-Supramolecule #3: Human RAD51
| Supramolecule | Name: Human RAD51 / type: complex / ID: 3 / Parent: 1 / Macromolecule list: #2 |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
-Macromolecule #1: DNA (5'-D(P*TP*TP*TP*TP*TP*TP*TP*TP*T)-3')
| Macromolecule | Name: DNA (5'-D(P*TP*TP*TP*TP*TP*TP*TP*TP*T)-3') / type: dna / ID: 1 / Number of copies: 1 / Classification: DNA |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 2.692778 KDa |
| Sequence | String: (DT)(DT)(DT)(DT)(DT)(DT)(DT)(DT)(DT) |
-Macromolecule #2: DNA repair protein RAD51 homolog 1
| Macromolecule | Name: DNA repair protein RAD51 homolog 1 / type: protein_or_peptide / ID: 2 / Number of copies: 3 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 37.008074 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: MAMQMQLEAN ADTSVEEESF GPQPISRLEQ CGINANDVKK LEEAGFHTVE AVAYAPKKEL INIKGISEAK ADKILAEAAK LVPMGFTTA TEFHQRRSEI IQITTGSKEL DKLLQGGIET GSITEMFGEF RTGKTQICHT LAVTCQLPID RGGGEGKAMY I DTEGTFRP ...String: MAMQMQLEAN ADTSVEEESF GPQPISRLEQ CGINANDVKK LEEAGFHTVE AVAYAPKKEL INIKGISEAK ADKILAEAAK LVPMGFTTA TEFHQRRSEI IQITTGSKEL DKLLQGGIET GSITEMFGEF RTGKTQICHT LAVTCQLPID RGGGEGKAMY I DTEGTFRP ERLLAVAERY GLSGSDVLDN VAYARAFNTD HQTQLLYQAS AMMVESRYAL LIVDSATALY RTDYSGRGEL SA RQMHLAR FLRMLLRLAD EFGVAVVITN QVVAQVDGAA MFAADPKKPI GGNIIAHAST TRLYLRKGRG ETRICQIYDS PCL PEAEAM FAINADGVGD AKD UniProtKB: DNA repair protein RAD51 homolog 1 |
-Macromolecule #3: MAGNESIUM ION
| Macromolecule | Name: MAGNESIUM ION / type: ligand / ID: 3 / Number of copies: 3 / Formula: MG |
|---|---|
| Molecular weight | Theoretical: 24.305 Da |
-Macromolecule #4: 4-bromanyl-N-(4-bromophenyl)-3-[(phenylmethyl)sulfamoyl]benzamide
| Macromolecule | Name: 4-bromanyl-N-(4-bromophenyl)-3-[(phenylmethyl)sulfamoyl]benzamide type: ligand / ID: 4 / Number of copies: 3 / Formula: J46 |
|---|---|
| Molecular weight | Theoretical: 524.226 Da |
| Chemical component information | 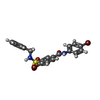 ChemComp-J46: |
-Macromolecule #5: PHOSPHOAMINOPHOSPHONIC ACID-ADENYLATE ESTER
| Macromolecule | Name: PHOSPHOAMINOPHOSPHONIC ACID-ADENYLATE ESTER / type: ligand / ID: 5 / Number of copies: 3 / Formula: ANP |
|---|---|
| Molecular weight | Theoretical: 506.196 Da |
| Chemical component information |  ChemComp-ANP: |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | helical reconstruction |
| Aggregation state | filament |
- Sample preparation
Sample preparation
| Buffer | pH: 7.5 |
|---|---|
| Vitrification | Cryogen name: ETHANE |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Image recording | Film or detector model: FEI FALCON II (4k x 4k) / Average electron dose: 48.0 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD / Nominal defocus max: 3.5 µm / Nominal defocus min: 1.0 µm |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
- Image processing
Image processing
| Final reconstruction | Applied symmetry - Helical parameters - Δz: 15.8 Å Applied symmetry - Helical parameters - Δ&Phi: 56.7 ° Applied symmetry - Helical parameters - Axial symmetry: C1 (asymmetric) Resolution.type: BY AUTHOR / Resolution: 2.97 Å / Resolution method: FSC 0.143 CUT-OFF / Number images used: 92403 |
|---|---|
| Startup model | Type of model: EMDB MAP EMDB ID: |
| Final angle assignment | Type: NOT APPLICABLE |
 Movie
Movie Controller
Controller


















 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)





















