+ Open data
Open data
- Basic information
Basic information
| Entry | Database: EMDB / ID: EMD-2986 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | The structure of the COPI coat linkage I | |||||||||
 Map data Map data | Reconstruction of the COPI coat linkage I | |||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | COPI / coatomer / coated vesicles | |||||||||
| Function / homology |  Function and homology information Function and homology informationCOPI-coated vesicle membrane / cerebellar Purkinje cell layer maturation / Synthesis of PIPs at the plasma membrane / protein localization to axon / VxPx cargo-targeting to cilium / protein localization to cell leading edge / Synthesis of PIPs at the Golgi membrane / protein localization to Golgi membrane / regulation of Golgi organization / pancreatic juice secretion ...COPI-coated vesicle membrane / cerebellar Purkinje cell layer maturation / Synthesis of PIPs at the plasma membrane / protein localization to axon / VxPx cargo-targeting to cilium / protein localization to cell leading edge / Synthesis of PIPs at the Golgi membrane / protein localization to Golgi membrane / regulation of Golgi organization / pancreatic juice secretion / COPI-coated vesicle / trans-Golgi Network Vesicle Budding / organelle membrane contact site / Intra-Golgi traffic / COPI-mediated anterograde transport / COPI vesicle coat / Golgi vesicle transport / COPI-dependent Golgi-to-ER retrograde traffic / COPI-mediated anterograde transport / COPI-dependent Golgi-to-ER retrograde traffic / positive regulation of mitochondrial fusion / organelle transport along microtubule / regulation of fatty acid metabolic process / establishment of Golgi localization / Golgi to plasma membrane transport / intra-Golgi vesicle-mediated transport / retrograde vesicle-mediated transport, Golgi to endoplasmic reticulum / positive regulation of mitochondrial fission / endoplasmic reticulum-Golgi intermediate compartment / pigmentation / endoplasmic reticulum to Golgi vesicle-mediated transport / vesicle-mediated transport / Neutrophil degranulation / small monomeric GTPase / adult locomotory behavior / macroautophagy / intracellular protein transport / establishment of localization in cell / hormone activity / protein transport / growth cone / Golgi membrane / axon / neuronal cell body / GTPase activity / mRNA binding / GTP binding / structural molecule activity / endoplasmic reticulum / Golgi apparatus / extracellular space / nucleoplasm / plasma membrane / cytoplasm / cytosol Similarity search - Function | |||||||||
| Biological species |   | |||||||||
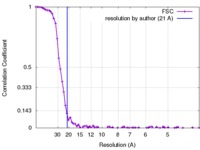
| Method | subtomogram averaging / cryo EM / Resolution: 21.0 Å | |||||||||
 Authors Authors | Dodonova SO / Diestelkoetter-Bachert P / von Appen A / Hagen WJH / Beck R / Beck M / Wieland F / Briggs JAG | |||||||||
 Citation Citation |  Journal: Science / Year: 2015 Journal: Science / Year: 2015Title: VESICULAR TRANSPORT. A structure of the COPI coat and the role of coat proteins in membrane vesicle assembly. Authors: S O Dodonova / P Diestelkoetter-Bachert / A von Appen / W J H Hagen / R Beck / M Beck / F Wieland / J A G Briggs /  Abstract: Transport of material within cells is mediated by trafficking vesicles that bud from one cellular compartment and fuse with another. Formation of a trafficking vesicle is driven by membrane coats ...Transport of material within cells is mediated by trafficking vesicles that bud from one cellular compartment and fuse with another. Formation of a trafficking vesicle is driven by membrane coats that localize cargo and polymerize into cages to bend the membrane. Although extensive structural information is available for components of these coats, the heterogeneity of trafficking vesicles has prevented an understanding of how complete membrane coats assemble on the membrane. We combined cryo-electron tomography, subtomogram averaging, and cross-linking mass spectrometry to derive a complete model of the assembled coat protein complex I (COPI) coat involved in traffic between the Golgi and the endoplasmic reticulum. The highly interconnected COPI coat structure contradicted the current "adaptor-and-cage" understanding of coated vesicle formation. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Movie |
 Movie viewer Movie viewer |
|---|---|
| Structure viewer | EM map:  SurfView SurfView Molmil Molmil Jmol/JSmol Jmol/JSmol |
| Supplemental images |
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_2986.map.gz emd_2986.map.gz | 28.6 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-2986-v30.xml emd-2986-v30.xml emd-2986.xml emd-2986.xml | 13 KB 13 KB | Display Display |  EMDB header EMDB header |
| FSC (resolution estimation) |  emd_2986_fsc.xml emd_2986_fsc.xml | 8.5 KB | Display |  FSC data file FSC data file |
| Images |  emd_2986.jpg emd_2986.jpg | 178.3 KB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-2986 http://ftp.pdbj.org/pub/emdb/structures/EMD-2986 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-2986 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-2986 | HTTPS FTP |
-Related structure data
| Related structure data |  5a1vMC  2985C  2987C  2988C  2989C  5a1uC  5a1wC  5a1xC  5a1yC M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_2986.map.gz / Format: CCP4 / Size: 29.8 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_2986.map.gz / Format: CCP4 / Size: 29.8 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Reconstruction of the COPI coat linkage I | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 2.019 Å | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Density |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
CCP4 map header:
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
-Supplemental data
- Sample components
Sample components
-Entire : COPI coat linkage I on the membrane
| Entire | Name: COPI coat linkage I on the membrane |
|---|---|
| Components |
|
-Supramolecule #1000: COPI coat linkage I on the membrane
| Supramolecule | Name: COPI coat linkage I on the membrane / type: sample / ID: 1000 Details: The COPI coated vesicles were formed in an in vitro reconstituted budding reaction, which was plunge-frozen without further purification. Number unique components: 2 |
|---|
-Macromolecule #1: Coat protein 1
| Macromolecule | Name: Coat protein 1 / type: protein_or_peptide / ID: 1 / Name.synonym: COPI / Recombinant expression: Yes |
|---|---|
| Source (natural) | Organism:  |
| Molecular weight | Theoretical: 560 KDa |
| Recombinant expression | Organism:  |
| Sequence | InterPro: Coatomer subunit alpha, Coatomer beta' subunit (COPB2), Coatomer, epsilon subunit, Coatomer beta subunit (COPB1), Coatomer delta subunit, Coatomer gamma subunit, AP complex, mu/sigma subunit |
-Macromolecule #2: ADP-ribosylation factor 1
| Macromolecule | Name: ADP-ribosylation factor 1 / type: protein_or_peptide / ID: 2 / Name.synonym: Arf1 / Recombinant expression: Yes |
|---|---|
| Source (natural) | Organism:  |
| Molecular weight | Theoretical: 20 KDa |
| Recombinant expression | Organism:  |
| Sequence | UniProtKB: ADP-ribosylation factor 1 / InterPro: Small GTPase superfamily, ARF type |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | subtomogram averaging |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Buffer | pH: 7.4 / Details: 50 mM HEPES, 50 mM KAc, 1mM MgCl2 |
|---|---|
| Grid | Details: C-Flat Multihole 3C-50 grids glow discharged |
| Vitrification | Cryogen name: ETHANE / Chamber humidity: 85 % / Instrument: HOMEMADE PLUNGER Method: The grids were glow discharged for 1 min at 20 mA. 5 ul of 10 nm fiducial gold was added to 40 ul of reaction mix. The reaction mix with the in vitro formed coated vesicles was applied to a ...Method: The grids were glow discharged for 1 min at 20 mA. 5 ul of 10 nm fiducial gold was added to 40 ul of reaction mix. The reaction mix with the in vitro formed coated vesicles was applied to a grid. The grid was blotted for 12 seconds at room temperature before plunging |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Temperature | Min: 89 K / Max: 91 K / Average: 90 K |
| Specialist optics | Energy filter - Name: GATAN GIF 2002 / Energy filter - Lower energy threshold: 0.0 eV / Energy filter - Upper energy threshold: 20.0 eV |
| Date | Feb 7, 2014 |
| Image recording | Category: CCD / Film or detector model: GATAN MULTISCAN / Average electron dose: 45 e/Å2 |
| Electron beam | Acceleration voltage: 200 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD / Cs: 2.7 mm / Nominal defocus max: 4.0 µm / Nominal defocus min: 1.5 µm / Nominal magnification: 42000 |
| Sample stage | Specimen holder model: FEI TITAN KRIOS AUTOGRID HOLDER / Tilt series - Axis1 - Min angle: -45 ° / Tilt series - Axis1 - Max angle: 60 ° |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
+ Image processing
Image processing
-Atomic model buiding 1
| Initial model | PDB ID: Chain - #0 - Chain ID: A / Chain - #1 - Chain ID: B |
|---|---|
| Software | Name:  Chimera Chimera |
| Details | The crystal structure (chains A and B) was fitted into the EM map using global search option in Chimera software. |
| Refinement | Space: REAL / Protocol: RIGID BODY FIT / Target criteria: cross-correlation |
| Output model |  PDB-5a1v: |
 Movie
Movie Controller
Controller






















 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)