[English] 日本語
 Yorodumi
Yorodumi- EMDB-27076: Cryo-EM asymmetric reconstruction of the EPEC H6 bacterial flagel... -
+ Open data
Open data
- Basic information
Basic information
| Entry |  | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | Cryo-EM asymmetric reconstruction of the EPEC H6 bacterial flagellar filament Normal Waveform | |||||||||
 Map data Map data | ||||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | Bacterial flagellum / flagellin / bacterial motility / supercoiling / STRUCTURAL PROTEIN | |||||||||
| Function / homology |  Function and homology information Function and homology informationbacterial-type flagellum / structural molecule activity / extracellular region Similarity search - Function | |||||||||
| Biological species |  | |||||||||
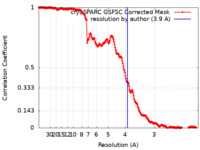
| Method | single particle reconstruction / cryo EM / Resolution: 3.9 Å | |||||||||
 Authors Authors | Kreutzberger MAB / Chatterjee S / Frankel G / Egelman EH | |||||||||
| Funding support |  United States, 1 items United States, 1 items
| |||||||||
 Citation Citation |  Journal: Cell / Year: 2022 Journal: Cell / Year: 2022Title: Convergent evolution in the supercoiling of prokaryotic flagellar filaments. Authors: Mark A B Kreutzberger / Ravi R Sonani / Junfeng Liu / Sharanya Chatterjee / Fengbin Wang / Amanda L Sebastian / Priyanka Biswas / Cheryl Ewing / Weili Zheng / Frédéric Poly / Gad Frankel / ...Authors: Mark A B Kreutzberger / Ravi R Sonani / Junfeng Liu / Sharanya Chatterjee / Fengbin Wang / Amanda L Sebastian / Priyanka Biswas / Cheryl Ewing / Weili Zheng / Frédéric Poly / Gad Frankel / B F Luisi / Chris R Calladine / Mart Krupovic / Birgit E Scharf / Edward H Egelman /    Abstract: The supercoiling of bacterial and archaeal flagellar filaments is required for motility. Archaeal flagellar filaments have no homology to their bacterial counterparts and are instead homologs of ...The supercoiling of bacterial and archaeal flagellar filaments is required for motility. Archaeal flagellar filaments have no homology to their bacterial counterparts and are instead homologs of bacterial type IV pili. How these prokaryotic flagellar filaments, each composed of thousands of copies of identical subunits, can form stable supercoils under torsional stress is a fascinating puzzle for which structural insights have been elusive. Advances in cryoelectron microscopy (cryo-EM) make it now possible to directly visualize the basis for supercoiling, and here, we show the atomic structures of supercoiled bacterial and archaeal flagellar filaments. For the bacterial flagellar filament, we identify 11 distinct protofilament conformations with three broad classes of inter-protomer interface. For the archaeal flagellar filament, 10 protofilaments form a supercoil geometry supported by 10 distinct conformations, with one inter-protomer discontinuity creating a seam inside of the curve. Our results suggest that convergent evolution has yielded stable superhelical geometries that enable microbial locomotion. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Supplemental images |
|---|
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_27076.map.gz emd_27076.map.gz | 70.9 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-27076-v30.xml emd-27076-v30.xml emd-27076.xml emd-27076.xml | 18.7 KB 18.7 KB | Display Display |  EMDB header EMDB header |
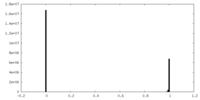
| FSC (resolution estimation) |  emd_27076_fsc.xml emd_27076_fsc.xml | 20.6 KB | Display |  FSC data file FSC data file |
| Images |  emd_27076.png emd_27076.png | 25.8 KB | ||
| Masks |  emd_27076_msk_1.map emd_27076_msk_1.map | 926.9 MB |  Mask map Mask map | |
| Filedesc metadata |  emd-27076.cif.gz emd-27076.cif.gz | 6 KB | ||
| Others |  emd_27076_half_map_1.map.gz emd_27076_half_map_1.map.gz emd_27076_half_map_2.map.gz emd_27076_half_map_2.map.gz | 859 MB 859.1 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-27076 http://ftp.pdbj.org/pub/emdb/structures/EMD-27076 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-27076 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-27076 | HTTPS FTP |
-Related structure data
| Related structure data |  8cyeMC  8cviC  8cwmC  8cxmC C: citing same article ( M: atomic model generated by this map |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|
- Map
Map
| File |  Download / File: emd_27076.map.gz / Format: CCP4 / Size: 926.9 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_27076.map.gz / Format: CCP4 / Size: 926.9 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 1.08 Å | ||||||||||||||||||||||||||||||||||||
| Density |
| ||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
|
-Supplemental data
-Mask #1
| File |  emd_27076_msk_1.map emd_27076_msk_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
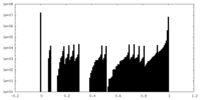
| Density Histograms |
-Half map: #2
| File | emd_27076_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Half map: #1
| File | emd_27076_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
| Density Histograms |
- Sample components
Sample components
-Entire : Bacterial flagellar filaments
| Entire | Name: Bacterial flagellar filaments |
|---|---|
| Components |
|
-Supramolecule #1: Bacterial flagellar filaments
| Supramolecule | Name: Bacterial flagellar filaments / type: complex / ID: 1 / Parent: 0 / Macromolecule list: all |
|---|---|
| Source (natural) | Organism:  |
-Macromolecule #1: Flagellin
| Macromolecule | Name: Flagellin / type: protein_or_peptide / ID: 1 / Number of copies: 22 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  |
| Molecular weight | Theoretical: 56.342008 KDa |
| Sequence | String: MAQVINTNSL SLITQNNINK NQSALSSSIE RLSSGLRINS AKDDAAGQAI ANRFTSNIKG LTQAARNAND GISVAQTTEG ALSEINNNL QRIRELTVQA STGTNSDSDL DSIQDEIKSR LDEIDRVSGQ TQFNGVNVLA KDGSMKIQVG ANDGQTITID L KKIDSDTL ...String: MAQVINTNSL SLITQNNINK NQSALSSSIE RLSSGLRINS AKDDAAGQAI ANRFTSNIKG LTQAARNAND GISVAQTTEG ALSEINNNL QRIRELTVQA STGTNSDSDL DSIQDEIKSR LDEIDRVSGQ TQFNGVNVLA KDGSMKIQVG ANDGQTITID L KKIDSDTL GLNGFNVNGK GETANTAATL KDMSGFTAAA APGGTVGVTQ YTDKSAVASS VDILNAVAGA DGNKVTTSAD VG FGTPAAA VTYTYNKDTN SYSAASDDIS SANLAAFLNP QARDTTKATV TIGGKDQDVN IDKSGNLTAA DDGAVLYMDA TGN LTKNNA GGDTQATLAK VATATGAKAA TIQTDKGTFT SDGTAFDGAS MSIDANTFAN AVKNDTYTAT VGAKTYSVTT GSAA ADTAY MSNGVLSDTP PTYYAQADGS ITTTEDAAAG KLVYKGSDGK LTTDTTSKAE STSDPLAALD DAISQIDKFR SSLGA VQNR LDSAVTNLNN TTTNLSEAQS RIQDADYATE VSNMSKAQII QQAGNSVLAK ANQVPQQVLS LLQG UniProtKB: Flagellin |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | filament |
- Sample preparation
Sample preparation
| Buffer | pH: 7.4 |
|---|---|
| Vitrification | Cryogen name: ETHANE |
- Electron microscopy
Electron microscopy
| Microscope | TFS KRIOS |
|---|---|
| Image recording | Film or detector model: GATAN K3 (6k x 4k) / Average electron dose: 50.0 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD / Nominal defocus max: 2.5 µm / Nominal defocus min: 1.2 µm |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
 Movie
Movie Controller
Controller











 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)