[English] 日本語
 Yorodumi
Yorodumi- EMDB-26611: Composite cryo-EM density map of the 8-nm repeat of the human spe... -
+ Open data
Open data
- Basic information
Basic information
| Entry |  | |||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Title | Composite cryo-EM density map of the 8-nm repeat of the human sperm tip singlet microtubule | |||||||||||||||||||||
 Map data Map data | ||||||||||||||||||||||
 Sample Sample |
| |||||||||||||||||||||
 Keywords Keywords | cilia / microtubule / sperm / cell motility / STRUCTURAL PROTEIN | |||||||||||||||||||||
| Function / homology |  Function and homology information Function and homology informationaxonemal microtubule doublet inner sheath / axoneme assembly / Post-chaperonin tubulin folding pathway / axonemal microtubule / Cilium Assembly / cytoskeleton-dependent intracellular transport / Carboxyterminal post-translational modifications of tubulin / organelle transport along microtubule / Microtubule-dependent trafficking of connexons from Golgi to the plasma membrane / forebrain morphogenesis ...axonemal microtubule doublet inner sheath / axoneme assembly / Post-chaperonin tubulin folding pathway / axonemal microtubule / Cilium Assembly / cytoskeleton-dependent intracellular transport / Carboxyterminal post-translational modifications of tubulin / organelle transport along microtubule / Microtubule-dependent trafficking of connexons from Golgi to the plasma membrane / forebrain morphogenesis / Sealing of the nuclear envelope (NE) by ESCRT-III / Intraflagellar transport / cerebellar cortex morphogenesis / Formation of tubulin folding intermediates by CCT/TriC / glial cell differentiation / dentate gyrus development / neuron projection arborization / flagellated sperm motility / Gap junction assembly / Prefoldin mediated transfer of substrate to CCT/TriC / Kinesins / Assembly and cell surface presentation of NMDA receptors / response to L-glutamate / pyramidal neuron differentiation / COPI-independent Golgi-to-ER retrograde traffic / centrosome cycle / COPI-dependent Golgi-to-ER retrograde traffic / natural killer cell mediated cytotoxicity / smoothened signaling pathway / regulation of synapse organization / ciliary base / startle response / Recycling pathway of L1 / motor behavior / microtubule polymerization / response to tumor necrosis factor / MHC class I protein binding / locomotory exploration behavior / response to mechanical stimulus / sperm flagellum / RHO GTPases activate IQGAPs / microtubule-based process / Hedgehog 'off' state / intercellular bridge / COPI-mediated anterograde transport / Activation of AMPK downstream of NMDARs / cytoplasmic microtubule / condensed chromosome / Loss of Nlp from mitotic centrosomes / Loss of proteins required for interphase microtubule organization from the centrosome / Recruitment of mitotic centrosome proteins and complexes / MHC class II antigen presentation / homeostasis of number of cells within a tissue / Mitotic Prometaphase / acrosomal vesicle / Recruitment of NuMA to mitotic centrosomes / Anchoring of the basal body to the plasma membrane / EML4 and NUDC in mitotic spindle formation / HSP90 chaperone cycle for steroid hormone receptors (SHR) in the presence of ligand / cellular response to calcium ion / AURKA Activation by TPX2 / Resolution of Sister Chromatid Cohesion / adult locomotory behavior / Translocation of SLC2A4 (GLUT4) to the plasma membrane / intracellular protein transport / neuromuscular junction / RHO GTPases Activate Formins / synapse organization / recycling endosome / PKR-mediated signaling / visual learning / cerebral cortex development / structural constituent of cytoskeleton / microtubule cytoskeleton organization / memory / neuron migration / cytoplasmic ribonucleoprotein granule / HCMV Early Events / Aggrephagy / calcium-dependent protein binding / azurophil granule lumen / The role of GTSE1 in G2/M progression after G2 checkpoint / mitotic spindle / Separation of Sister Chromatids / unfolded protein binding / Regulation of PLK1 Activity at G2/M Transition / mitotic cell cycle / double-stranded RNA binding / extracellular vesicle / microtubule cytoskeleton / neuron apoptotic process / gene expression / microtubule binding / Hydrolases; Acting on acid anhydrides; Acting on GTP to facilitate cellular and subcellular movement / microtubule / cytoskeleton / cilium / ciliary basal body / hydrolase activity / protein heterodimerization activity Similarity search - Function | |||||||||||||||||||||
| Biological species |  Homo sapiens (human) Homo sapiens (human) | |||||||||||||||||||||
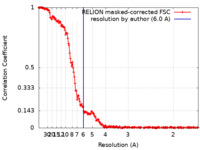
| Method | single particle reconstruction / cryo EM / Resolution: 6.0 Å | |||||||||||||||||||||
 Authors Authors | Gui M / Croft JT / Zabeo D / Acharya V / Kollman JM / Burgoyne T / Hoog JL / Brown A | |||||||||||||||||||||
| Funding support |  United States, United States,  Sweden, 6 items Sweden, 6 items
| |||||||||||||||||||||
 Citation Citation |  Journal: Proc Natl Acad Sci U S A / Year: 2022 Journal: Proc Natl Acad Sci U S A / Year: 2022Title: SPACA9 is a lumenal protein of human ciliary singlet and doublet microtubules. Authors: Miao Gui / Jacob T Croft / Davide Zabeo / Vajradhar Acharya / Justin M Kollman / Thomas Burgoyne / Johanna L Höög / Alan Brown /    Abstract: The cilium-centrosome complex contains triplet, doublet, and singlet microtubules. The lumenal surfaces of each microtubule within this diverse array are decorated by microtubule inner proteins ...The cilium-centrosome complex contains triplet, doublet, and singlet microtubules. The lumenal surfaces of each microtubule within this diverse array are decorated by microtubule inner proteins (MIPs). Here, we used single-particle cryo-electron microscopy methods to build atomic models of two types of human ciliary microtubule: the doublet microtubules of multiciliated respiratory cells and the distal singlet microtubules of monoflagellated human spermatozoa. We discover that SPACA9 is a polyspecific MIP capable of binding both microtubule types. SPACA9 forms intralumenal striations in the B tubule of respiratory doublet microtubules and noncontinuous spirals in sperm singlet microtubules. By acquiring new and reanalyzing previous cryo-electron tomography data, we show that SPACA9-like intralumenal striations are common features of different microtubule types in animal cilia. Our structures provide detailed references to help rationalize ciliopathy-causing mutations and position cryo-EM as a tool for the analysis of samples obtained directly from ciliopathy patients. | |||||||||||||||||||||
| History |
|
- Structure visualization
Structure visualization
| Supplemental images |
|---|
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_26611.map.gz emd_26611.map.gz | 373.7 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-26611-v30.xml emd-26611-v30.xml emd-26611.xml emd-26611.xml | 35.5 KB 35.5 KB | Display Display |  EMDB header EMDB header |
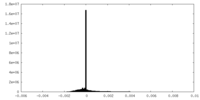
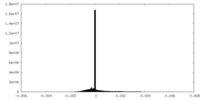
| FSC (resolution estimation) |  emd_26611_fsc.xml emd_26611_fsc.xml | 17.1 KB | Display |  FSC data file FSC data file |
| Images |  emd_26611.png emd_26611.png | 133.6 KB | ||
| Masks |  emd_26611_msk_1.map emd_26611_msk_1.map | 421.9 MB |  Mask map Mask map | |
| Filedesc metadata |  emd-26611.cif.gz emd-26611.cif.gz | 6.7 KB | ||
| Others |  emd_26611_additional_1.map.gz emd_26611_additional_1.map.gz emd_26611_additional_2.map.gz emd_26611_additional_2.map.gz emd_26611_additional_3.map.gz emd_26611_additional_3.map.gz emd_26611_additional_4.map.gz emd_26611_additional_4.map.gz emd_26611_additional_5.map.gz emd_26611_additional_5.map.gz emd_26611_additional_6.map.gz emd_26611_additional_6.map.gz emd_26611_additional_7.map.gz emd_26611_additional_7.map.gz emd_26611_additional_8.map.gz emd_26611_additional_8.map.gz emd_26611_half_map_1.map.gz emd_26611_half_map_1.map.gz emd_26611_half_map_2.map.gz emd_26611_half_map_2.map.gz | 48.6 MB 47.7 MB 337.9 MB 84.2 MB 47.6 MB 47.6 MB 48.3 MB 48.6 MB 340.4 MB 340.4 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-26611 http://ftp.pdbj.org/pub/emdb/structures/EMD-26611 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-26611 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-26611 | HTTPS FTP |
-Related structure data
| Related structure data |  7un1MC  7ungC M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_26611.map.gz / Format: CCP4 / Size: 421.9 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_26611.map.gz / Format: CCP4 / Size: 421.9 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 0.843 Å | ||||||||||||||||||||||||||||||||||||
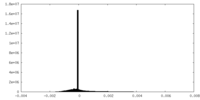
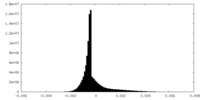
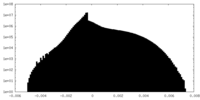
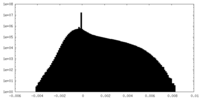
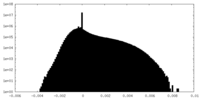
| Density |
| ||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
|
-Supplemental data
+Mask #1
+Additional map: local refined map
+Additional map: local refined map
+Additional map: unsharpened consensus map
+Additional map: unsharpened stitched map
+Additional map: local refined map
+Additional map: local refined map
+Additional map: local refined map
+Additional map: local refined map
+Half map: #2
+Half map: #1
- Sample components
Sample components
-Entire : Singlet microtubule and associated SPACA9
| Entire | Name: Singlet microtubule and associated SPACA9 |
|---|---|
| Components |
|
-Supramolecule #1: Singlet microtubule and associated SPACA9
| Supramolecule | Name: Singlet microtubule and associated SPACA9 / type: complex / ID: 1 / Parent: 0 / Macromolecule list: #1-#3 |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
-Macromolecule #1: Sperm acrosome-associated protein 9
| Macromolecule | Name: Sperm acrosome-associated protein 9 / type: protein_or_peptide / ID: 1 / Number of copies: 33 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 25.208977 KDa |
| Sequence | String: MNEVKESLRS IEQKYKLFQQ QQLTFTAALE HCRENAHDKI RPISSIGQVQ SYMEHYCNSS TDRRVLLMFL DICSELNKLC QHFEAVHSG TPVTNNLLEK CKTLVSQSND LSSLRAKYPH DVVNHLSCDE ARNHYGGVVS LIPLILDLMK EWIAHSEKLP R KVLQHVSE ...String: MNEVKESLRS IEQKYKLFQQ QQLTFTAALE HCRENAHDKI RPISSIGQVQ SYMEHYCNSS TDRRVLLMFL DICSELNKLC QHFEAVHSG TPVTNNLLEK CKTLVSQSND LSSLRAKYPH DVVNHLSCDE ARNHYGGVVS LIPLILDLMK EWIAHSEKLP R KVLQHVSE PQAHQESTRG AARPAQAIGT QPRATKHKCR QLTKASLKPR GCSKPPWRPP GGKL UniProtKB: Sperm acrosome-associated protein 9 |
-Macromolecule #2: Tubulin beta-4B chain
| Macromolecule | Name: Tubulin beta-4B chain / type: protein_or_peptide / ID: 2 / Number of copies: 38 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 49.877824 KDa |
| Sequence | String: MREIVHLQAG QCGNQIGAKF WEVISDEHGI DPTGTYHGDS DLQLERINVY YNEATGGKYV PRAVLVDLEP GTMDSVRSGP FGQIFRPDN FVFGQSGAGN NWAKGHYTEG AELVDSVLDV VRKEAESCDC LQGFQLTHSL GGGTGSGMGT LLISKIREEY P DRIMNTFS ...String: MREIVHLQAG QCGNQIGAKF WEVISDEHGI DPTGTYHGDS DLQLERINVY YNEATGGKYV PRAVLVDLEP GTMDSVRSGP FGQIFRPDN FVFGQSGAGN NWAKGHYTEG AELVDSVLDV VRKEAESCDC LQGFQLTHSL GGGTGSGMGT LLISKIREEY P DRIMNTFS VVPSPKVSDT VVEPYNATLS VHQLVENTDE TYCIDNEALY DICFRTLKLT TPTYGDLNHL VSATMSGVTT CL RFPGQLN ADLRKLAVNM VPFPRLHFFM PGFAPLTSRG SQQYRALTVP ELTQQMFDAK NMMAACDPRH GRYLTVAAVF RGR MSMKEV DEQMLNVQNK NSSYFVEWIP NNVKTAVCDI PPRGLKMSAT FIGNSTAIQE LFKRISEQFT AMFRRKAFLH WYTG EGMDE MEFTEAESNM NDLVSEYQQY QDATAEEEGE FEEEAEEEVA UniProtKB: Tubulin beta-4B chain |
-Macromolecule #3: Tubulin alpha-1A chain
| Macromolecule | Name: Tubulin alpha-1A chain / type: protein_or_peptide / ID: 3 / Number of copies: 38 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 50.188441 KDa |
| Sequence | String: MRECISIHVG QAGVQIGNAC WELYCLEHGI QPDGQMPSDK TIGGGDDSFN TFFSETGAGK HVPRAVFVDL EPTVIDEVRT GTYRQLFHP EQLITGKEDA ANNYARGHYT IGKEIIDLVL DRIRKLADQC TGLQGFLVFH SFGGGTGSGF TSLLMERLSV D YGKKSKLE ...String: MRECISIHVG QAGVQIGNAC WELYCLEHGI QPDGQMPSDK TIGGGDDSFN TFFSETGAGK HVPRAVFVDL EPTVIDEVRT GTYRQLFHP EQLITGKEDA ANNYARGHYT IGKEIIDLVL DRIRKLADQC TGLQGFLVFH SFGGGTGSGF TSLLMERLSV D YGKKSKLE FSIYPAPQVS TAVVEPYNSI LTTHTTLEHS DCAFMVDNEA IYDICRRNLD IERPTYTNLN RLIGQIVSSI TA SLRFDGA LNVDLTEFQT NLVPYPRIHF PLATYAPVIS AEKAYHEQLS VAEITNACFE PANQMVKCDP RHGKYMACCL LYR GDVVPK DVNAAIATIK TKRTIQFVDW CPTGFKVGIN YQPPTVVPGG DLAKVQRAVC MLSNTTAIAE AWARLDHKFD LMYA KRAFV HWYVGEGMEE GEFSEAREDM AALEKDYEEV GVDSVEGEGE EEGEEY UniProtKB: Tubulin alpha-1A chain |
-Macromolecule #4: GUANOSINE-5'-DIPHOSPHATE
| Macromolecule | Name: GUANOSINE-5'-DIPHOSPHATE / type: ligand / ID: 4 / Number of copies: 38 / Formula: GDP |
|---|---|
| Molecular weight | Theoretical: 443.201 Da |
| Chemical component information |  ChemComp-GDP: |
-Macromolecule #5: GUANOSINE-5'-TRIPHOSPHATE
| Macromolecule | Name: GUANOSINE-5'-TRIPHOSPHATE / type: ligand / ID: 5 / Number of copies: 38 / Formula: GTP |
|---|---|
| Molecular weight | Theoretical: 523.18 Da |
| Chemical component information |  ChemComp-GTP: |
-Macromolecule #6: MAGNESIUM ION
| Macromolecule | Name: MAGNESIUM ION / type: ligand / ID: 6 / Number of copies: 38 / Formula: MG |
|---|---|
| Molecular weight | Theoretical: 24.305 Da |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | filament |
- Sample preparation
Sample preparation
| Buffer | pH: 7.4 |
|---|---|
| Grid | Model: EMS Lacey Carbon / Material: COPPER / Mesh: 200 / Pretreatment - Type: GLOW DISCHARGE |
| Vitrification | Cryogen name: ETHANE |
| Details | Filaments |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Image recording | Film or detector model: GATAN K3 BIOQUANTUM (6k x 4k) / Average electron dose: 40.0 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD / Nominal defocus max: 3.0 µm / Nominal defocus min: 1.0 µm |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
 Movie
Movie Controller
Controller


































 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)