[English] 日本語
 Yorodumi
Yorodumi- EMDB-25649: Helical Reconstruction of the C-terminal Half of Leucine Rich Rep... -
+ Open data
Open data
- Basic information
Basic information
| Entry |  | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | Helical Reconstruction of the C-terminal Half of Leucine Rich Repeat Kinase 2 (LRRK2) (I2020T) bound to 11-protofilament microtubule in presence of MLi-2 kinase inhibitor | |||||||||
 Map data Map data | LRRK2 RCKW I2020T bound to 11-pf microtubule with MLi-2. No helical symmetry on final reconstruction due to symmetry mismatch between LRRK2 and the microtubule. | |||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | parkinson's disease / microtubule / kinase / gtpase / CYTOSOLIC PROTEIN | |||||||||
| Biological species |  Homo sapiens (human) / Homo sapiens (human) /  | |||||||||
| Method | helical reconstruction / cryo EM / Resolution: 18.0 Å | |||||||||
 Authors Authors | Matyszewski M / Leschziner AE | |||||||||
| Funding support |  United States, 1 items United States, 1 items
| |||||||||
 Citation Citation |  Journal: Nat Struct Mol Biol / Year: 2022 Journal: Nat Struct Mol Biol / Year: 2022Title: Structural basis for Parkinson's disease-linked LRRK2's binding to microtubules. Authors: David M Snead / Mariusz Matyszewski / Andrea M Dickey / Yu Xuan Lin / Andres E Leschziner / Samara L Reck-Peterson /  Abstract: Leucine-rich repeat kinase 2 (LRRK2) is one of the most commonly mutated genes in familial Parkinson's disease (PD). Under some circumstances, LRRK2 co-localizes with microtubules in cells, an ...Leucine-rich repeat kinase 2 (LRRK2) is one of the most commonly mutated genes in familial Parkinson's disease (PD). Under some circumstances, LRRK2 co-localizes with microtubules in cells, an association enhanced by PD mutations. We report a cryo-EM structure of the catalytic half of LRRK2, containing its kinase, in a closed conformation, and GTPase domains, bound to microtubules. We also report a structure of the catalytic half of LRRK1, which is closely related to LRRK2 but is not linked to PD. Although LRRK1's structure is similar to that of LRRK2, we find that LRRK1 does not interact with microtubules. Guided by these structures, we identify amino acids in LRRK2's GTPase that mediate microtubule binding; mutating them disrupts microtubule binding in vitro and in cells, without affecting LRRK2's kinase activity. Our results have implications for the design of therapeutic LRRK2 kinase inhibitors. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Supplemental images |
|---|
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_25649.map.gz emd_25649.map.gz | 105.4 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-25649-v30.xml emd-25649-v30.xml emd-25649.xml emd-25649.xml | 20.1 KB 20.1 KB | Display Display |  EMDB header EMDB header |
| FSC (resolution estimation) |  emd_25649_fsc.xml emd_25649_fsc.xml | 15.2 KB | Display |  FSC data file FSC data file |
| Images |  emd_25649.png emd_25649.png | 87.4 KB | ||
| Filedesc metadata |  emd-25649.cif.gz emd-25649.cif.gz | 7 KB | ||
| Others |  emd_25649_half_map_1.map.gz emd_25649_half_map_1.map.gz emd_25649_half_map_2.map.gz emd_25649_half_map_2.map.gz | 105.9 MB 106 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-25649 http://ftp.pdbj.org/pub/emdb/structures/EMD-25649 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-25649 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-25649 | HTTPS FTP |
-Related structure data
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|
- Map
Map
| File |  Download / File: emd_25649.map.gz / Format: CCP4 / Size: 134.6 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_25649.map.gz / Format: CCP4 / Size: 134.6 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | LRRK2 RCKW I2020T bound to 11-pf microtubule with MLi-2. No helical symmetry on final reconstruction due to symmetry mismatch between LRRK2 and the microtubule. | ||||||||||||||||||||||||||||||||||||
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 2.32 Å | ||||||||||||||||||||||||||||||||||||
| Density |
| ||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
|
-Supplemental data
-Half map: Half-map 2
| File | emd_25649_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Half-map 2 | ||||||||||||
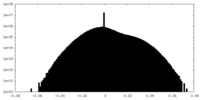
| Projections & Slices |
| ||||||||||||
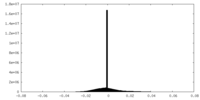
| Density Histograms |
-Half map: Half-map 1
| File | emd_25649_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Half-map 1 | ||||||||||||
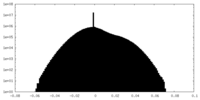
| Projections & Slices |
| ||||||||||||
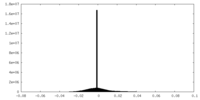
| Density Histograms |
- Sample components
Sample components
-Entire : LRRK2RCKW filament bound to a 11-pf microtubule with MLi-2 present
| Entire | Name: LRRK2RCKW filament bound to a 11-pf microtubule with MLi-2 present |
|---|---|
| Components |
|
-Supramolecule #1: LRRK2RCKW filament bound to a 11-pf microtubule with MLi-2 present
| Supramolecule | Name: LRRK2RCKW filament bound to a 11-pf microtubule with MLi-2 present type: complex / ID: 1 / Parent: 0 / Macromolecule list: all |
|---|
-Macromolecule #1: Catalytic C-terminus of Leucine Rich Repeat Kinase 2
| Macromolecule | Name: Catalytic C-terminus of Leucine Rich Repeat Kinase 2 / type: protein_or_peptide / ID: 1 Details: I2020T mutation present Residues 1327-2527 of LRRK2 Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Recombinant expression | Organism:  |
| Sequence | String: KKAVPYNRMK LMIVGNTGSG KTTLLQQLMK TKKSDLGMQS ATVGIDVKDW PIQIRDKRKR DLVLNVWDF AGREEFYSTH PHFMTQRALY LAVYDLSKGQ AEVDAMKPWL FNIKARASSS P VILVGTHL DVSDEKQRKA CMSKITKELL NKRGFPAIRD YHFVNATEES ...String: KKAVPYNRMK LMIVGNTGSG KTTLLQQLMK TKKSDLGMQS ATVGIDVKDW PIQIRDKRKR DLVLNVWDF AGREEFYSTH PHFMTQRALY LAVYDLSKGQ AEVDAMKPWL FNIKARASSS P VILVGTHL DVSDEKQRKA CMSKITKELL NKRGFPAIRD YHFVNATEES DALAKLRKTI IN ESLNFKI RDQLVVGQLI PDCYVELEKI ILSERKNVPI EFPVIDRKRL LQLVRENQLQ LDE NELPHA VHFLNESGVL LHFQDPALQL SDLYFVEPKW LCKIMAQILT VKVEGCPKHP KGII SRRDV EKFLSKKRKF PKNYMSQYFK LLEKFQIALP IGEEYLLVPS SLSDHRPVIE LPHCE NSEI IIRLYEMPYF PMGFWSRLIN RLLEISPYML SGRERALRPN RMYWRQGIYL NWSPEA YCL VGSEVLDNHP ESFLKITVPS CRKGCILLGQ VVDHIDSLME EWFPGLLEID ICGEGET LL KKWALYSFND GEEHQKILLD DLMKKAEEGD LLVNPDQPRL TIPISQIAPD LILADLPR N IMLNNDELEF EQAPEFLLGD GSFGSVYRAA YEGEEVAVKI FNKHTSLRLL RQELVVLCH LHHPSLISLL AAGIRPRMLV MELASKGSLD RLLQQDKASL TRTLQHRIAL HVADGLRYLH SAMIIYRDL KPHNVLLFTL YPNAAIIAKI ADYGTAQYCC RMGIKTSEGT PGFRAPEVAR G NVIYNQQA DVYSFGLLLY DILTTGGRIV EGLKFPNEFD ELEIQGKLPD PVKEYGCAPW PM VEKLIKQ CLKENPQERP TSAQVFDILN SAELVCLTRR ILLPKNVIVE CMVATHHNSR NAS IWLGCG HTDRGQLSFL DLNTEGYTSE EVADSRILCL ALVHLPVEKE SWIVSGTQSG TLLV INTED GKKRHTLEKM TDSVTCLYCN SFSKQSKQKN FLLVGTADGK LAIFEDKTVK LKGAA PLKI LNIGNVSTPL MCLSESTNST ERNVMWGGCG TKIFSFSNDF TIQKLIETRT SQLFSY AAF SDSNIITVVV DTALYIAKQN SPVVEVWDKK TEKLCGLIDC VHFLREVMVK ENKESKH KM SYSGRVKTLC LQKNTALWIG TGGGHILLLD LSTRRLIRVI YNFCNSVRVM MTAQLGSL K NVMLVLGYNR KNTEGTQKQK EIQSCLTVWD INLPHEVQNL EKHIEVRKEL AEKMRRTSV E |
-Macromolecule #2: Tubulin alpha
| Macromolecule | Name: Tubulin alpha / type: protein_or_peptide / ID: 2 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  |
| Sequence | String: MRECISIHVG QAGVQIGNAC WELYCLEHGI QPDGQMPSDK TIGGGDDSFN TFFSETGAGK HVPRAVFVD LEPTVIDEVR TGTYRQLFHP EQLITGKEDA ANNYARGHYT IGKEIIDLVL D RIRKLADQ CTGLQGFLVF HSFGGGTGSG FTSLLMERLS VDYGKKSKLE ...String: MRECISIHVG QAGVQIGNAC WELYCLEHGI QPDGQMPSDK TIGGGDDSFN TFFSETGAGK HVPRAVFVD LEPTVIDEVR TGTYRQLFHP EQLITGKEDA ANNYARGHYT IGKEIIDLVL D RIRKLADQ CTGLQGFLVF HSFGGGTGSG FTSLLMERLS VDYGKKSKLE FSIYPAPQVS TA VVEPYNS ILTTHTTLEH SDCAFMVDNE AIYDICRRNL DIERPTYTNL NRLIGQIVSS ITA SLRFDG ALNVDLTEFQ TNLVPYPRIH FPLATYAPVI SAEKAYHEQL SVAEITNACF EPAN QMVKC DPRHGKYMAC CLLYRGDVVP KDVNAAIATI KTKRTIQFVD WCPTGFKVGI NYQPP TVVP GGDLAKVQRA VCMLSNTTAI AEAWARLDHK FDLMYAKRAF VHWYVGEGME EGEFSE ARE DMAALEKDYE EVGVDSVEGE GEEEGEEY |
-Macromolecule #3: Tublin beta
| Macromolecule | Name: Tublin beta / type: protein_or_peptide / ID: 3 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  |
| Sequence | String: MREIVHIQIG QCGNQIGAKF WEVIGEEHGI DWAGSYCGDS ALQLERISVY YNEAHGKKYV PRAVLVDLE PGTMDSIRSS RVGALFQPDS FVHGNSGAGN NWAKGYYTEG AELVDRVLDA V RTEAEGCD CLQGFQLVHS LGGGTGSGMG TLLLGKIREE YPDRILNSFS ...String: MREIVHIQIG QCGNQIGAKF WEVIGEEHGI DWAGSYCGDS ALQLERISVY YNEAHGKKYV PRAVLVDLE PGTMDSIRSS RVGALFQPDS FVHGNSGAGN NWAKGYYTEG AELVDRVLDA V RTEAEGCD CLQGFQLVHS LGGGTGSGMG TLLLGKIREE YPDRILNSFS VMPSPKVSDT VV EPYNAVL ALHQLVLNSD ACFCIDNEAL YDICFRTLRL STPTYGDLNH LVSLTMSGIT TSL RFPGQL NADLRKLAVN MVPFPRLHFF MPGFAPLTAQ GSQQYRALTV AELTQQMFDA RNTM AACDP RRGRYLTVAC IFRGRMSTKE VDEQLLNVQT RNSSCFVEWI PNNVKVAVCD IPPRG LSMA ATFIGNNTAI QELFSRISEH FSAMFKRKAF VHWYTGEGMD INEFTEAESN IQDLVS EYQ QFQDARADVE EEEIGGEAEV EPADKEH |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | helical reconstruction |
| Aggregation state | filament |
- Sample preparation
Sample preparation
| Buffer | pH: 7.4 Component:
Details: This is the final dilution buffer. The incubation buffer consisted of 1x BRB80, 10% glycerol, 1mM DTT, 1mM GTP, 1mM MgCl2, 10 uM taxol, and 5 uM MLi-2. Sample was diluted 3-fold right before ...Details: This is the final dilution buffer. The incubation buffer consisted of 1x BRB80, 10% glycerol, 1mM DTT, 1mM GTP, 1mM MgCl2, 10 uM taxol, and 5 uM MLi-2. Sample was diluted 3-fold right before freezing with the final buffer. | ||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Grid | Model: EMS Lacey Carbon / Material: COPPER / Mesh: 300 / Support film - Material: CARBON / Support film - topology: LACEY / Pretreatment - Type: GLOW DISCHARGE / Pretreatment - Time: 45 sec. / Details: EMS (LC-300) | ||||||||||||||||||
| Vitrification | Cryogen name: ETHANE / Chamber humidity: 100 % / Chamber temperature: 277 K | ||||||||||||||||||
| Details | 4.5 uM of LRRK2RCKW was allowed to incubate with 2.25 uM of tubulin dimer, causing both to co-polymerize. 5 uM of MLi-2 was present as well. The sample was diluted 3-fold right before freezing (1.5 uM LRRK2RCKW concentration final). |
- Electron microscopy
Electron microscopy
| Microscope | FEI TALOS ARCTICA |
|---|---|
| Image recording | Film or detector model: GATAN K2 SUMMIT (4k x 4k) / Detector mode: COUNTING / Number grids imaged: 1 / Average exposure time: 10.0 sec. / Average electron dose: 55.0 e/Å2 / Details: 250 ms frames |
| Electron beam | Acceleration voltage: 200 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | C2 aperture diameter: 70.0 µm / Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD / Cs: 2.7 mm / Nominal defocus max: 1.5 µm / Nominal defocus min: 1.5 µm / Nominal magnification: 36000 |
| Sample stage | Cooling holder cryogen: NITROGEN |
| Experimental equipment |  Model: Talos Arctica / Image courtesy: FEI Company |
 Movie
Movie Controller
Controller













 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)